Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy
- PMID: 19652709
- PMCID: PMC2714967
- DOI: 10.1371/journal.pone.0006498
Human mesenchymal stem cells self-renew and differentiate according to a deterministic hierarchy
Abstract
Background: Mesenchymal progenitor cells (MPCs) have been isolated from a variety of connective tissues, and are commonly called "mesenchymal stem cells" (MSCs). A stem cell is defined as having robust clonal self-renewal and multilineage differentiation potential. Accordingly, the term "MSC" has been criticised, as there is little data demonstrating self-renewal of definitive single-cell-derived (SCD) clonal populations from a mesenchymal cell source.
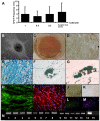
Methodology/principal findings: Here we show that a tractable MPC population, human umbilical cord perivascular cells (HUCPVCs), was capable of multilineage differentiation in vitro and, more importantly, contributed to rapid connective tissue healing in vivo by producing bone, cartilage and fibrous stroma. Furthermore, HUCPVCs exhibit a high clonogenic frequency, allowing us to isolate definitive SCD parent and daughter clones from mixed gender suspensions as determined by Y-chromosome fluorescent in situ hybridization.
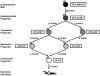
Conclusions/significance: Analysis of the multilineage differentiation capacity of SCD parent clones and daughter clones enabled us to formulate a new hierarchical schema for MSC self-renewal and differentiation in which a self-renewing multipotent MSC gives rise to more restricted self-renewing progenitors that gradually lose differentiation potential until a state of complete restriction to the fibroblast is reached.
Conflict of interest statement
Figures
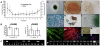



References
-
- Friedenstein AJ, Gorskaja JF, Kulagina NN. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp Hematol. 1976;4:267–274. - PubMed
-
- Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56:289–301. - PubMed
-
- Kuznetsov SA, Friedenstein AJ, Robey PG. Factors required for bone marrow stromal fibroblast colony formation in vitro. Br J Haematol. 1997;97:561–570. - PubMed
-
- Kuznetsov SA, Krebsbach PH, Satomura K, Kerr J, Riminucci M, et al. Single-colony derived strains of human marrow stromal fibroblasts form bone after transplantation in vivo. J Bone Miner Res. 1997;12:1335–1347. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

