Malarial hemozoin is a Nalp3 inflammasome activating danger signal
- PMID: 19652710
- PMCID: PMC2714977
- DOI: 10.1371/journal.pone.0006510
Malarial hemozoin is a Nalp3 inflammasome activating danger signal
Abstract
Background: Characteristic symptoms of malaria include recurrent fever attacks and neurodegeneration, signs that are also found in patients with a hyperactive Nalp3 inflammasome. Plasmodium species produce a crystal called hemozoin that is generated by detoxification of heme after hemoglobin degradation in infected red blood cells. Thus, we hypothesized that hemozoin could activate the Nalp3 inflammasome, due to its particulate nature reminiscent of other inflammasome-activating agents.
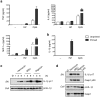
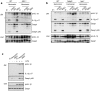
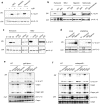
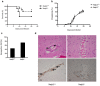
Methodology/principal findings: We found that hemozoin acts as a proinflammatory danger signal that activates the Nalp3 inflammasome, causing the release of IL-1beta. Similar to other Nalp3-activating particles, hemozoin activity is blocked by inhibiting phagocytosis, K(+) efflux and NADPH oxidase. In vivo, intraperitoneal injection of hemozoin results in acute peritonitis, which is impaired in Nalp3-, caspase-1- and IL-1R-deficient mice. Likewise, the pathogenesis of cerebral malaria is dampened in Nalp3-deficient mice infected with Plasmodium berghei sporozoites, while parasitemia remains unchanged.
Significance/conclusions: The potent pro-inflammatory effect of hemozoin through inflammasome activation may possibly be implicated in plasmodium-associated pathologies such as cerebral malaria.
Conflict of interest statement
Figures



References
-
- Silvie O, Mota MM, Matuschewski K, Prudencio M. Interactions of the malaria parasite and its mammalian host. Curr Opin Microbiol. 2008;11:352–359. - PubMed
-
- Idro R, Jenkins NE, Newton CR. Pathogenesis, clinical features, and neurological outcome of cerebral malaria. Lancet Neurol. 2005;4:827–840. - PubMed
-
- Beeson JG, Osier FH, Engwerda CR. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol. 2008;24:578–584. - PubMed
-
- Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. - PubMed
-
- Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

