Neuroprotective effect of long-term NDI1 gene expression in a chronic mouse model of Parkinson disorder
- PMID: 19653878
- PMCID: PMC2939843
- DOI: 10.1089/rej.2009.0854
Neuroprotective effect of long-term NDI1 gene expression in a chronic mouse model of Parkinson disorder
Abstract
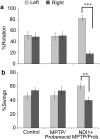
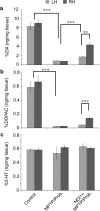
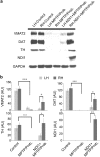
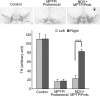
Previously, we showed that the internal rotenone-insensitive nicotinamide adenine dinucleotide (NADH)-quinone oxidoreductase (NDI1) gene from Saccharomyces cerevisiae (baker's yeast) can be successfully inserted into the mitochondria of mice and rats and the expressed enzyme was found to be fully functional. In this study, we investigated the ability of the Ndi1 enzyme to protect the dopaminergic neurons in a chronic mouse model of Parkinson disorder. After expression of the NDI1 gene in the unilateral substantia nigra of male C57BL/6 mice for 8 months, a chronic Parkinsonian model was created by administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) with probenecid and evaluated using neurochemical and behavioral responses 1-4 weeks post-MPTP/probenecid injection. We showed that expression of Ndi1 was able to significantly prevent the loss of dopamine and tyrosine hydroxylase as well as the dopaminergic transporters in the striatum of the chronic Parkinsonian mice. Behavioral assessment based on a methamphetamine-induced rotation test and spontaneous swing test further supported neurological preservation in the NDI1-treated Parkinsonian mice. The data presented in this study demonstrate a protective effect of the NDI1 gene in dopaminergic neurons, suggesting its therapeutic potential for Parkinson-like disorders.
Figures

References
-
- During MJ. Leone P. Targets for gene therapy of Parkinson's disease: growth factors, signal transduction, and promoters. Exp Neurol. 1997;144:74–81. - PubMed
-
- Eberling JL. Jagust WJ. Christine CW. Starr P. Larson P. Bankiewicz KS. Aminoff MJ. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. - PubMed
-
- Fiandaca M. Forsayeth J. Bankiewicz K. Current status of gene therapy trials for Parkinson's disease. Exp Neurol. 2007;209:51–57. - PubMed
-
- Kaplitt MG. Feigin A. Tang C. Fitzsimons HL. Mattis P. Lawlor PA. Bland RJ. Young D. Strybing K. Eidelberg D. During MJ. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. - PubMed
-
- Marks WJ., Jr. Ostrem JL. Verhagen L. Starr PA. Larson PS. Bakay RA. Taylor R. Cahn-Weiner DA. Stoessl AJ. Olanow CW. Bartus RT. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

