TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A
- PMID: 19654323
- PMCID: PMC2781400
- DOI: 10.1074/jbc.M109.010074
TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A
Abstract
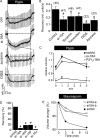
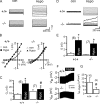
All vertebrate cells regulate their cell volume by activating chloride channels of unknown molecular identity, thereby activating regulatory volume decrease. We show that the Ca(2+)-activated Cl(-) channel TMEM16A together with other TMEM16 proteins are activated by cell swelling through an autocrine mechanism that involves ATP release and binding to purinergic P2Y(2) receptors. TMEM16A channels are activated by ATP through an increase in intracellular Ca(2+) and a Ca(2+)-independent mechanism engaging extracellular-regulated protein kinases (ERK1/2). The ability of epithelial cells to activate a Cl(-) conductance upon cell swelling, and to decrease their cell volume (regulatory volume decrease) was dependent on TMEM16 proteins. Activation of I(Cl,swell) was reduced in the colonic epithelium and in salivary acinar cells from mice lacking expression of TMEM16A. Thus TMEM16 proteins appear to be a crucial component of epithelial volume-regulated Cl(-) channels and may also have a function during proliferation and apoptotic cell death.
Figures





References
-
- Stutzin A., Hoffmann E. K. (2006) Acta Physiol. (Oxf.) 187, 27–42 - PubMed
-
- Okada Y., Maeno E., Shimizu T., Manabe K., Mori S., Nabekura T. (2004) Pflugers Arch. 448, 287–295 - PubMed
-
- Lang F., Busch G. L., Ritter M., Völkl H., Waldegger S., Gulbins E., Häussinger D. (1998) Physiol. Rev. 78, 247–306 - PubMed
-
- Lang F., Madlung J., Bock J., Lükewille U., Kaltenbach S., Lang K. S., Belka C., Wagner C. A., Lang H. J., Gulbins E., Lepple-Wienhues A. (2000) Pflugers Arch. 440, 902–907 - PubMed
-
- Hoffmann E. K., Simonsen L. O., Lambert I. H. (1984) J. Membr. Biol. 78, 211–222 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

