Arylacetamide deacetylase attenuates fatty-acid-induced triacylglycerol accumulation in rat hepatoma cells
- PMID: 19654421
- PMCID: PMC2803239
- DOI: 10.1194/jlr.M000596
Arylacetamide deacetylase attenuates fatty-acid-induced triacylglycerol accumulation in rat hepatoma cells
Abstract
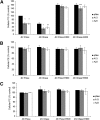
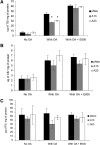
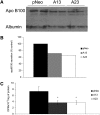
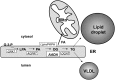
Mobilization of hepatic triacylglycerol stores provides substrates for mitochondrial beta-oxidation and assembly of VLDLs; however, the identity of lipolytic enzymes involved in the regulation of this process remains largely unknown. Arylacetamide deacetylase (AADA) shares homology with hormone-sensitive lipase and therefore could potentially participate in hepatic lipid metabolism, including the regulation of hepatic triacylglycerol levels. We have established McArdle-RH7777 (rat hepatoma) cell lines stably expressing mouse AADA cDNA and performed metabolic labeling as well as lipid mass analyses. Expression of AADA cDNA in McArdle-RH7777 cells significantly reduced intracellular triacylglycerol levels and apolipoprotein B secretion and increased fatty acid oxidation. These results suggest that fatty acids released by AADA-mediated hydrolysis of lipids are channeled for -oxidation rather than for the assembly of lipoproteins.
Figures



References
-
- Yang L. Y., Kuksis A., Myher J. J., Steiner G. 1995. Origin of triacylglycerol moiety of plasma very low density lipoproteins in the rat: structural studies. J. Lipid Res. 36: 125–136. - PubMed
-
- Lankester D. L., Brown A. M., Zammit V. A. 1998. Use of cytosolic triacylglycerol hydrolysis products and of exogenous fatty acid for the synthesis of triacylglycerol secreted by cultured rat hepatocytes. J. Lipid Res. 39: 1889–1895. - PubMed
-
- Boren J., Rustaeus S., Olofsson S. O. 1994. Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem. 269: 25879–25888. - PubMed
-
- Rustaeus S., Stillemark P., Lindberg K., Gordon D., Olofsson S. O. 1998. The microsomal triglyceride transfer protein catalyzes the post-translational assembly of apolipoprotein B-100 very low density lipoprotein in McA-RH7777 cells. J. Biol. Chem. 273: 5196–5203. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

