Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women
- PMID: 19654872
- PMCID: PMC2716517
- DOI: 10.1371/journal.pone.0006518
Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women
Abstract
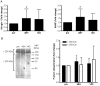
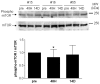
Advancements in animal models and cell culture techniques have been invaluable in the elucidation of the molecular mechanisms that regulate muscle atrophy. However, few studies have examined muscle atrophy in humans using modern experimental techniques. The purpose of this study was to examine changes in global gene transcription during immobilization-induced muscle atrophy in humans and then explore the effects of the most prominent transcriptional alterations on protein expression and function. Healthy men and women (N = 24) were subjected to two weeks of unilateral limb immobilization, with muscle biopsies obtained before, after 48 hours (48 H) and 14 days (14 D) of immobilization. Muscle cross sectional area (approximately 5%) and strength (10-20%) were significantly reduced in men and women (approximately 5% and 10-20%, respectively) after 14 D of immobilization. Micro-array analyses of total RNA extracted from biopsy samples at 48 H and 14 D uncovered 575 and 3,128 probes, respectively, which were significantly altered during immobilization. As a group, genes involved in mitochondrial bioenergetics and carbohydrate metabolism were predominant features at both 48 H and 14 D, with genes involved in protein synthesis and degradation significantly down-regulated and up-regulated, respectively, at 14 D of muscle atrophy. There was also a significant decrease in the protein content of mitochondrial cytochrome c oxidase, and the enzyme activity of cytochrome c oxidase and citrate synthase after 14 D of immobilization. Furthermore, protein ubiquitination was significantly increased at 48 H but not 14 D of immobilization. These results suggest that transcriptional and post-transcriptional suppression of mitochondrial processes is sustained throughout 14 D of immobilization, while protein ubiquitination plays an early but transient role in muscle atrophy following short-term immobilization in humans.
Conflict of interest statement
Figures



References
-
- Lecker SH, Jagoe RT, Gilbert A, Gomes M, Baracos V, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. - PubMed
-
- Booth FW, Nicholson WF, Watson PA. Influence of muscle use on protein synthesis and degradation. Exerc Sport Sci Rev. 1982;10:27–48. - PubMed
-
- Zinna EM, Yarasheski KE. Exercise treatment to counteract protein wasting of chronic diseases. Curr Opin Clin Nutr Metab Care. 2003;6:87–93. - PubMed
-
- Mansoor O, Bazin JE, Beaufrere B, Schoeffler P. [Catabolic aspects of cranial trauma]. Ann Fr Anesth Reanim. 1998;17:180–185. - PubMed
-
- Baracos VE. Management of muscle wasting in cancer-associated cachexia: understanding gained from experimental studies. Cancer. 2001;92:1669–1677. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

