Apoptosis of CD4+ CD25(high) T cells in type 1 diabetes may be partially mediated by IL-2 deprivation
- PMID: 19654878
- PMCID: PMC2716541
- DOI: 10.1371/journal.pone.0006527
Apoptosis of CD4+ CD25(high) T cells in type 1 diabetes may be partially mediated by IL-2 deprivation
Abstract
Background: Type 1 diabetes (T1D) is a T-cell mediated autoimmune disease targeting the insulin-producing pancreatic beta cells. Naturally occurring FOXP3(+)CD4(+)CD25(high) regulatory T cells (T(regs)) play an important role in dominant tolerance, suppressing autoreactive CD4(+) effector T cell activity. Previously, in both recent-onset T1D patients and beta cell antibody-positive at-risk individuals, we observed increased apoptosis and decreased function of polyclonal T(regs) in the periphery. Our objective here was to elucidate the genes and signaling pathways triggering apoptosis in T(regs) from T1D subjects.
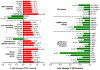
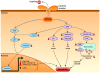
Principal findings: Gene expression profiles of unstimulated T(regs) from recent-onset T1D (n = 12) and healthy control subjects (n = 15) were generated. Statistical analysis was performed using a Bayesian approach that is highly efficient in determining differentially expressed genes with low number of replicate samples in each of the two phenotypic groups. Microarray analysis showed that several cytokine/chemokine receptor genes, HLA genes, GIMAP family genes and cell adhesion genes were downregulated in T(regs) from T1D subjects, relative to control subjects. Several downstream target genes of the AKT and p53 pathways were also upregulated in T1D subjects, relative to controls. Further, expression signatures and increased apoptosis in T(regs) from T1D subjects partially mirrored the response of healthy T(regs) under conditions of IL-2 deprivation. CD4(+) effector T-cells from T1D subjects showed a marked reduction in IL-2 secretion. This could indicate that prior to and during the onset of disease, T(regs) in T1D may be caught up in a relatively deficient cytokine milieu.
Conclusions: In summary, expression signatures in T(regs) from T1D subjects reflect a cellular response that leads to increased sensitivity to apoptosis, partially due to cytokine deprivation. Further characterization of these signaling cascades should enable the detection of genes that can be targeted for restoring T(reg) function in subjects predisposed to T1D.
Conflict of interest statement
Figures




References
-
- Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85:291–297. - PubMed
-
- Donath MY, Storling J, Maedler K, Mandrup-Poulsen T. Inflammatory mediators and islet beta-cell failure: a link between type 1 and type 2 diabetes. J Mol Med. 2003;81:455–470. - PubMed
-
- Bacchetta R, Gambineri E, Roncarolo MG. Role of regulatory T cells and FOXP3 in human diseases. J Allergy Clin Immunol. 2007;120:227–235; quiz 236–227. - PubMed
-
- Putnam AL, Vendrame F, Dotta F, Gottlieb PA. CD4+CD25high regulatory T cells in human autoimmune diabetes. J Autoimmun. 2005;24:55–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

