Afferent nerve regulation of bladder function in health and disease
- PMID: 19655106
- PMCID: PMC3383010
- DOI: 10.1007/978-3-540-79090-7_4
Afferent nerve regulation of bladder function in health and disease
Abstract
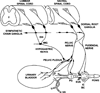
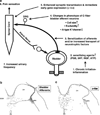
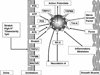
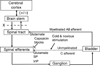
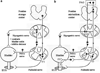
The afferent innervation of the urinary bladder consists primarily of small myelinated (Adelta) and unmyelinated (C-fiber) axons that respond to chemical and mechanical stimuli. Immunochemical studies indicate that bladder afferent neurons synthesize several putative neurotransmitters, including neuropeptides, glutamic acid, aspartic acid, and nitric oxide. The afferent neurons also express various types of receptors and ion channels, including transient receptor potential channels, purinergic, muscarinic, endothelin, neurotrophic factor, and estrogen receptors. Patch-clamp recordings in dissociated bladder afferent neurons and recordings of bladder afferent nerve activity have revealed that activation of many of these receptors enhances neuronal excitability. Afferent nerves can respond to chemicals present in urine as well as chemicals released in the bladder wall from nerves, smooth muscle, inflammatory cells, and epithelial cells lining the bladder lumen. Pathological conditions alter the chemical and electrical properties of bladder afferent pathways, leading to urinary urgency, increased voiding frequency, nocturia, urinary incontinence, and pain. Neurotrophic factors have been implicated in the pathophysiological mechanisms underlying the sensitization of bladder afferent nerves. Neurotoxins such as capsaicin, resiniferatoxin, and botulinum neurotoxin that target sensory nerves are useful in treating disorders of the lower urinary tract.
Figures

Similar articles
-
Changes in afferent activity after spinal cord injury.Neurourol Urodyn. 2010;29(1):63-76. doi: 10.1002/nau.20761. Neurourol Urodyn. 2010. PMID: 20025033 Free PMC article. Review.
-
Bladder hyperactivity and increased excitability of bladder afferent neurons associated with reduced expression of Kv1.4 alpha-subunit in rats with cystitis.Am J Physiol Regul Integr Comp Physiol. 2009 May;296(5):R1661-70. doi: 10.1152/ajpregu.91054.2008. Epub 2009 Mar 11. Am J Physiol Regul Integr Comp Physiol. 2009. PMID: 19279288 Free PMC article.
-
The role of capsaicin-sensitive C-fiber afferent pathways in the control of micturition in spinal-intact and spinal cord-injured mice.Am J Physiol Renal Physiol. 2017 Sep 1;313(3):F796-F804. doi: 10.1152/ajprenal.00097.2017. Epub 2017 Jun 21. Am J Physiol Renal Physiol. 2017. PMID: 28637786 Free PMC article.
-
Mechanisms of action of botulinum neurotoxins, β3-adrenergic receptor agonists, and PDE5 inhibitors in modulating detrusor function in overactive bladders: ICI-RS 2011.Neurourol Urodyn. 2012 Mar;31(3):300-8. doi: 10.1002/nau.21246. Epub 2012 Jan 24. Neurourol Urodyn. 2012. PMID: 22275187 Review.
-
Pharmacological and genetic analysis of mechanisms underlying detrusor overactivity in rats.Neurourol Urodyn. 2010;29(1):107-11. doi: 10.1002/nau.20746. Neurourol Urodyn. 2010. PMID: 20025014
Cited by
-
Urodynamic findings and vibegron effects on neurogenic lower urinary tract dysfunction caused by human T-cell leukemia virus type I-associated myelopathy/tropical spastic paraparesis.IJU Case Rep. 2024 Aug 29;7(6):438-441. doi: 10.1002/iju5.12773. eCollection 2024 Nov. IJU Case Rep. 2024. PMID: 39498183 Free PMC article.
-
Microbial colonization and ureteral stent-associated storage lower urinary tract symptoms: the forgotten piece of the puzzle?World J Urol. 2013 Jun;31(3):541-6. doi: 10.1007/s00345-012-0849-6. Epub 2012 Mar 4. World J Urol. 2013. PMID: 22391647
-
Chiropractic Care of an 8-Year-Old Girl With Nonorganic, Primary Nocturnal Enuresis: A Case Report.J Chiropr Med. 2016 Mar;15(1):47-52. doi: 10.1016/j.jcm.2016.02.002. Epub 2016 Feb 26. J Chiropr Med. 2016. PMID: 27069432 Free PMC article.
-
The KV 7 channel activator retigabine suppresses mouse urinary bladder afferent nerve activity without affecting detrusor smooth muscle K+ channel currents.J Physiol. 2019 Feb;597(3):935-950. doi: 10.1113/JP277021. Epub 2018 Dec 26. J Physiol. 2019. PMID: 30536555 Free PMC article.
-
PACAP/VIP and receptor characterization in micturition pathways in mice with overexpression of NGF in urothelium.J Mol Neurosci. 2010 Nov;42(3):378-89. doi: 10.1007/s12031-010-9384-3. Epub 2010 May 7. J Mol Neurosci. 2010. PMID: 20449688 Free PMC article.
References
-
- Andrade EL, Ferreira J, Andre E, Calixto JB. Contractile mechanisms coupled to TRPA1 receptor activation in rat urinary bladder. Biochem Pharmacol. 2006;72:104–114. - PubMed
-
- Apostolidis A, Fowler CJ. The use of botulinum neurotoxin type A (BoNTA) in urology. J Neural Transm. 2008;115:593–605. - PubMed
-
- Apostolidis A, Brady CM, Yiangou Y, Davis J, Fowler CJ, Anand P. Capsaicin receptor TRPV1 in urothelium of neurogenic human bladders and effect of intravesical resiniferatoxin. Urology. 2005a;65:400–405. - PubMed
-
- Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005b;174:977–982. - PubMed
-
- Apostolidis A, Dasgupta P, Fowler CJ. Proposed mechanism for the efficacy of injected botulinum toxin in the treatment of human detrusor overactivity. Eur Urol. 2006;49:644–650. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

