Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer
- PMID: 19655244
- PMCID: PMC4182952
- DOI: 10.1007/s10549-009-0495-x
Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer
Abstract
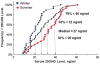
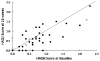
Vitamin D deficiency and insufficiency may contribute to musculoskeletal symptoms and bone loss observed in women taking aromatase inhibitors (AIs). This study was conducted to determine the prevalence of suboptimal vitamin D levels in women initiating adjuvant letrozole for breast cancer and to determine whether supplementation with 50,000 IU of vitamin D3 weekly could reduce musculoskeletal symptoms and fatigue in women who have suboptimal vitamin D levels. Sixty women about to begin an adjuvant AI were enrolled. Baseline 25OHD levels were obtained, and women completed symptom questionnaires. They were then started on letrozole, along with standard dose calcium and vitamin D. Four weeks later, women with baseline 25OHD levels </=40 ng/ml started additional vitamin D3 supplementation at 50,000 IU per week for 12 weeks. 25OHD levels were re-assessed at 4, 10, and 16 weeks; the questionnaires were repeated at weeks 4 and 16. At baseline, 63% of women exhibited vitamin D deficiency (<20 ng/ml) or insufficiency (20-31 ng/ml). 25OHD levels >40 ng/ml were achieved in all 42 subjects who received 12 weeks of supplementation with 50,000 IU vitamin D3 weekly, with no adverse effects. After 16 weeks of letrozole, more women with 25OHD levels >66 ng/ml (median level) reported no disability from joint pain than did women with levels <66 ng/ml (52 vs. 19%; P = 0.026). Vitamin D deficiency and insufficiency are prevalent in post-menopausal women initiating adjuvant AI. Vitamin D3 supplementation with 50,000 IU per week is safe, significantly increases 25OHD levels, and may reduce disability from AI-induced arthralgias.
Figures

References
-
- Baum M, Budzar AU, Cuzick J, et al. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet. 2002;359:2131–2139. - PubMed
-
- Howell A, Cuzick J, Baum M, et al. Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. - PubMed
-
- Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol. 2007;25:486–492. - PubMed
-
- Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. New Engl J Med. 2004;350:1081–1092. - PubMed
-
- Coombes RC, Kilburn LS, Snowdon CF, et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (intergroup exemestane study): a randomized controlled trial. Lancet. 2007;369:559–570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

