Activation of membrane cholesterol by 63 amphipaths
- PMID: 19655814
- PMCID: PMC2739890
- DOI: 10.1021/bi900951r
Activation of membrane cholesterol by 63 amphipaths
Abstract
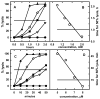
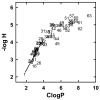
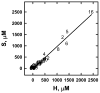
A few membrane-intercalating amphipaths have been observed to stimulate the interaction of cholesterol with cholesterol oxidase, saponin and cyclodextrin, presumably by displacing cholesterol laterally from its phospholipid complexes. We now report that this effect, referred to as cholesterol activation, occurs with dozens of other amphipaths, including alkanols, saturated and cis- and trans-unsaturated fatty acids, fatty acid methyl esters, sphingosine derivatives, terpenes, alkyl ethers, ketones, aromatics and cyclic alkyl derivatives. The apparent potency of the agents tested ranged from 3 microM to 7 mM and generally paralleled their octanol/water partition coefficients, except that relative potency declined for compounds with >10 carbons. Some small amphipaths activated cholesterol at a membrane concentration of approximately 3 mol per 100 mol of bilayer lipids, about equimolar with the cholesterol they displaced. Lysophosphatidylserine countered the effects of all these agents, consistent with its ability to reduce the pool of active membrane cholesterol. Various amphipaths stabilized red cells against the hemolysis elicited by cholesterol depletion, presumably by substituting for the extracted sterol. The number and location of cis and trans fatty acid unsaturations and the absolute stereochemistry of enantiomer pairs had only small effects on amphipath potency. Nevertheless, potency varied approximately 7-fold within a group of diverse agents with similar partition coefficients. We infer that a wide variety of amphipaths can displace membrane cholesterol by competing stoichiometrically but with only limited specificity for weak association with phospholipids. Any number of other drugs and experimental agents might do the same.
Figures





References
-
- Barenholz Y. Cholesterol and other membrane active sterols: from membrane evolution to “rafts”. Prog Lipid Res. 2002;41:1–5. - PubMed
-
- Leermakers FAM, Rabinovich AL. Interaction of cholesterol-like molecules in polyunsaturated phosphatidylcholine lipid bilayers as revealed by a self-consistent field theory. Physical Review E (Statistical, Nonlinear, and Soft Matter Physics) 2007;76:031904. - PubMed
-
- Yeagle PL. Cholesterol rotation in phospholipid vesicles as observed by 13C-NMR. Biochim Biophys Acta. 1981;640:263–273. - PubMed
-
- Westover E, Covey D. The enantiomer of cholesterol. J Membr Biol. 2004;202:61–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

