Critical androgen-sensitive periods of rat penis and clitoris development
- PMID: 19656234
- PMCID: PMC2816361
- DOI: 10.1111/j.1365-2605.2009.00978.x
Critical androgen-sensitive periods of rat penis and clitoris development
Abstract
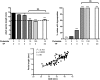
Androgen control of penis development/growth is unclear. In rats, androgen action in a foetal 'masculinisation programming window' (MPW; e15.5-e18.5)' predetermines penile length and hypospadias occurrence. This has implications for humans (e.g. micropenis). Our studies aimed to establish in rats when androgen action/administration affects development/growth of the penis and if deficits in MPW androgen action were rescuable postnatally. Thus, pregnant rats were treated with flutamide during the MPW +/- postnatal testosterone propionate (TP) treatment. To assess penile growth responsiveness, rats were treated with TP in various time windows (late foetal, neonatal through early puberty, puberty onset, or combinations thereof). Phallus length, weight, and morphology, hypospadias and anogenital distance (AGD) were measured in mid-puberty (d25) or adulthood (d90) in males and females, plus serum testosterone in adult males. MPW flutamide exposure reduced adult penile length and induced hypospadias dose-dependently; this was not rescued by postnatal TP treatment. In normal rats, foetal (e14.5-e21.5) TP exposure did not affect male penis size but increased female clitoral size. In males, TP exposure from postnatal d1-24 or at puberty (d15-24), increased penile length at d25, but not ultimately in adulthood. Foetal + postnatal TP (e14-postnatal d24) increased penile size at d25 but reduced it at d90 (due to reduced endogenous testosterone). In females, this treatment caused the biggest increase in adult clitoral size but, unlike in males, phallus size was unaffected by TP during puberty (d15-24). Postnatal TP treatment advanced penile histology at d25 to more resemble adult histology. AGD strongly correlated with final penis length. It is concluded that adult penile size depends critically on androgen action during the MPW but subsequent growth depends on later androgen exposure. Foetal and/or postnatal TP exposure does not increase adult penile size above its 'predetermined' length though its growth towards this maximum is advanced by peripubertal TP treatment.
Figures


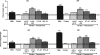
Similar articles
-
Androgen action in the masculinization programming window and development of male reproductive organs.Int J Androl. 2010 Apr;33(2):279-87. doi: 10.1111/j.1365-2605.2009.01005.x. Epub 2009 Nov 30. Int J Androl. 2010. PMID: 20002220
-
Relative importance of prenatal and postnatal androgen action in determining growth of the penis and anogenital distance in the rat before, during and after puberty.Int J Androl. 2011 Dec;34(6 Pt 2):e578-86. doi: 10.1111/j.1365-2605.2011.01175.x. Epub 2011 Jun 2. Int J Androl. 2011. PMID: 21631528
-
The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats.Int J Androl. 2012 Jun;35(3):330-9. doi: 10.1111/j.1365-2605.2011.01236.x. Epub 2012 Jan 17. Int J Androl. 2012. PMID: 22248293
-
Androgens and the masculinization programming window: human-rodent differences.Biochem Soc Trans. 2020 Aug 28;48(4):1725-1735. doi: 10.1042/BST20200200. Biochem Soc Trans. 2020. PMID: 32779695 Free PMC article. Review.
-
Development of the external genitalia: perspectives from the spotted hyena (Crocuta crocuta).Differentiation. 2014 Jan-Feb;87(1-2):4-22. doi: 10.1016/j.diff.2013.12.003. Epub 2014 Feb 28. Differentiation. 2014. PMID: 24582573 Free PMC article. Review.
Cited by
-
Anti-lysyl oxidase combined with a vacuum device induces penile lengthening by remodeling the tunica albuginea.Asian J Androl. 2020 Sep-Oct;22(5):485-492. doi: 10.4103/aja.aja_120_19. Asian J Androl. 2020. PMID: 31736474 Free PMC article.
-
Testosterone regulates keratin 33B expression in rat penis growth through androgen receptor signaling.Asian J Androl. 2014 Nov-Dec;16(6):817-23. doi: 10.4103/1008-682X.129935. Asian J Androl. 2014. PMID: 24994782 Free PMC article.
-
Diethylstilbestrol-induced mouse hypospadias: "window of susceptibility".Differentiation. 2016 Jan-Mar;91(1-3):1-18. doi: 10.1016/j.diff.2016.01.004. Epub 2016 Jan 20. Differentiation. 2016. PMID: 26810244 Free PMC article. Review.
-
HCG supplement did not accelerate tunica albuginea remodeling to facilitate penile growth.Sci Rep. 2023 Oct 2;13(1):16519. doi: 10.1038/s41598-023-38888-y. Sci Rep. 2023. PMID: 37783699 Free PMC article.
-
Hormonal and Molecular Regulation of Phallus Differentiation in a Marsupial Tammar Wallaby.Genes (Basel). 2020 Jan 16;11(1):106. doi: 10.3390/genes11010106. Genes (Basel). 2020. PMID: 31963388 Free PMC article. Review.
References
-
- Atanassova N, McKinnell C, Walker M, Turner KJ, Fisher JS, Morley M, Millar MR, Groome NP, Sharpe RM. Permanent effects of neonatal estrogen exposure in rats on reproductive hormone levels, Sertoli cell number and the efficiency of spermatogenesis in adulthood. Endocrinology. 1999;140:5364–5373. - PubMed
-
- Baskin LS, Sutherland RS, DiSandro MJ, Hayward SW, Lipschutz J, Cunha GR. The effect of testosterone on androgen receptors and human penile growth. Journal of Urology. 1997;158:1113–1118. - PubMed
-
- Bin-Abbas B, Conte FA, Grumbach MM, Kaplan SL. Congenital hypogonadotropic hypogonadism and micropenis: effect of testosterone treatment on adult penile size why sex reversal is not indicated. Journal of Pediatrics. 1999;134:579–583. - PubMed
-
- Boas M, Boisen KA, Virtanen HE, Kaleva M, Suomi AM, Schmidt IM, et al. Postnatal penile length and growth rate correlate to serum testosterone levels: a longitudinal study of 1962 normal boys. European Journal of Endocrinology. 2006;154:125–129. - PubMed
-
- Brown GR, Nevison CM, Fraser HM, Dixson AF. Manipulation of postnatal testosterone levels affects phallic and clitoral development in infant rhesus monkeys. International Journal of Andrology. 1999;22:119–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

