Podosome-like structures of non-invasive carcinoma cells are replaced in epithelial-mesenchymal transition by actin comet-embedded invadopodia
- PMID: 19656240
- PMCID: PMC3829022
- DOI: 10.1111/j.1582-4934.2009.00868.x
Podosome-like structures of non-invasive carcinoma cells are replaced in epithelial-mesenchymal transition by actin comet-embedded invadopodia
Erratum in
- J Cell Mol Med. 2010 Aug;14(8):2185
Abstract
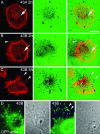
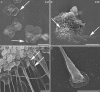
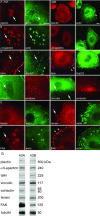
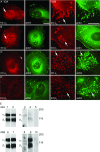
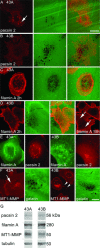
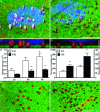
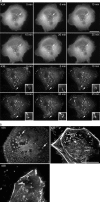
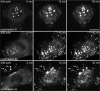
Podosomes and invadopodia are actin-based structures at the ventral cell membrane, which have a role in cell adhesion, migration and invasion. Little is known about the differences and dynamics underlying these structures. We studied podosome-like structures of oral squamous carcinoma cells and invadopodia of their invasive variant that has undergone a spontaneous epithelial-mesenchymal transition (EMT). In 3D imaging, podosomes were relatively large structures that enlarged in time, whereas invadopodia of invasive cells remained small, but were more numerous, degraded more extracellular matrix (ECM) and were morphologically strikingly different from podosomes. In live-cell imaging, highly dynamic, invadopodia-embedded actin tails were frequently released and rocketed through the cytoplasm. Resembling invadopodia, we found new club-ended cell extensions in EMT-experienced cells, which contained actin, cortactin, vinculin and MT1-matrix metalloproteinase. These dynamic cell extensions degraded ECM and, in field emission scanning electron microscopy, protruded from the dorsal cell membrane. Plectin, alphaII-spectrin, talin and focal adhesion kinase immunoreactivities were detected in podosome rings, whereas they were absent from invadopodia. Tensin potentially replaced talin in invadopodia. Integrin alpha(3)beta(1) surrounded both podosomes and invadopodia, whereas integrin alpha(v)beta(5) localized only to invadopodia heads. Pacsin 2, in conjunction with filamin A, was detected early in podosomes, whereas pacsin 2 was not found in invadopodia and filamin A showed delayed accumulation. Fluorescence recovery after photobleaching indicated faster reorganization of actin, cortactin and filamin A in podosomes compared to invadopodia. In conclusion, EMT affects the invasion machinery of oral squamous carcinoma cells. Non-invasive squamous carcinoma cells constitutively organize podosomes, whereas invasive cells form invadopodia. The club-ended cell extensions, or externalized invadopodia, are involved in ECM degradation and maintenance of contact to adhesion substrate and surrounding cells during invasion.
Figures


References
-
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat Rev Cancer. 2004;4:118–32. - PubMed
-
- Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. - PubMed
-
- Linder S. The matrix corroded: podosomes and invadopodia in extracellular matrix degradation. Trends Cell Biol. 2007;17:107–17. - PubMed
-
- Lehto VP, Hovi T, Vartio T, et al. Reorganization of cytoskeletal and contractile elements during transition of human monocytes into adherent macrophages. Lab Invest. 1982;47:391–9. - PubMed
-
- Tarone G, Cirillo D, Giancotti FG, et al. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

