cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake
- PMID: 19656393
- PMCID: PMC2731736
- DOI: 10.1186/1756-6606-2-26
cGMP-dependent protein kinase Ialpha associates with the antidepressant-sensitive serotonin transporter and dictates rapid modulation of serotonin uptake
Abstract
Background: The Na(+)/Cl(-)-dependent serotonin (5-hydroxytryptamine, 5-HT) transporter (SERT) is a critical element in neuronal 5-HT signaling, being responsible for the efficient elimination of 5-HT after release. SERTs are not only targets for exogenous addictive and therapeutic agents but also can be modulated by endogenous, receptor-linked signaling pathways. We have shown that neuronal A3 adenosine receptor activation leads to enhanced presynaptic 5-HT transport in vitro and an increased rate of SERT-mediated 5-HT clearance in vivo. SERT stimulation by A3 adenosine receptors derives from an elevation of cGMP and subsequent activation of both cGMP-dependent protein kinase (PKG) and p38 mitogen-activated protein kinase. PKG activators such as 8-Br-cGMP are known to lead to transporter phosphorylation, though how this modification supports SERT regulation is unclear.
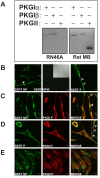
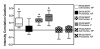
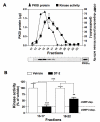
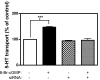
Results: In this report, we explore the kinase isoform specificity underlying the rapid stimulation of SERT activity by PKG activators. Using immortalized, rat serotonergic raphe neurons (RN46A) previously shown to support 8-Br-cGMP stimulation of SERT surface trafficking, we document expression of PKGI, and to a lower extent, PKGII. Quantitative analysis of staining profiles using permeabilized or nonpermeabilized conditions reveals that SERT colocalizes with PKGI in both intracellular and cell surface domains of RN46A cell bodies, and exhibits a more restricted, intracellular pattern of colocalization in neuritic processes. In the same cells, SERT demonstrates a lack of colocalization with PKGII in either intracellular or surface membranes. In keeping with the ability of the membrane permeant kinase inhibitor DT-2 to block 8-Br-cGMP stimulation of SERT, we found that DT-2 treatment eliminated cGMP-dependent kinase activity in PKGI-immunoreactive extracts resolved by liquid chromatography. Similarly, treatment of SERT-transfected HeLa cells with small interfering RNAs targeting endogenous PKGI eliminated 8-Br-cGMP-induced regulation of SERT activity. Co-immunoprecipitation studies show that, in transporter/kinase co-transfected cells, PKGIalpha specifically associates with hSERT.
Conclusion: Our findings provide evidence of a physical and compartmentalized association between SERT and PKGIalpha that supports rapid, 8-Br-cGMP-induced regulation of SERT. We discuss a model wherein SERT-associated PKGIalpha supports sequentially the mobilization of intracellular transporter-containing vesicles, leading to enhanced surface expression, and the production of catalytic-modulatory SERT phosphorylation, leading to a maximal enhancement of 5-HT clearance capacity.
Figures
References
-
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, Mossner R, Westphal H, Lesch KP. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine ("Ecstasy") in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. - PubMed
-
- Fabre V, Beaufour C, Evrard A, Rioux A, Hanoun N, Lesch KP, Murphy DL, Lanfumey L, Hamon M, Martres MP. Altered expression and functions of serotonin 5-HT1A and 5-HT1B receptors in knock-out mice lacking the 5-HT transporter. Eur J Neurosci. 2000;12:2299–2310. doi: 10.1046/j.1460-9568.2000.00126.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DA007390/DA/NIDA NIH HHS/United States
- R01 MH058921/MH/NIMH NIH HHS/United States
- R01 HL068891/HL/NHLBI NIH HHS/United States
- P50 MH078028/MH/NIMH NIH HHS/United States
- DK58277/DK/NIDDK NIH HHS/United States
- DA07390/DA/NIDA NIH HHS/United States
- DA007390-17S1/DA/NIDA NIH HHS/United States
- MH58921/MH/NIMH NIH HHS/United States
- MH078028/MH/NIMH NIH HHS/United States
- R01 DK040029/DK/NIDDK NIH HHS/United States
- HL68891/HL/NHLBI NIH HHS/United States
- DK40029/DK/NIDDK NIH HHS/United States
- R01 DK058277/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

