Imprinting regulates mammalian snoRNA-encoding chromatin decondensation and neuronal nucleolar size
- PMID: 19656775
- PMCID: PMC2766290
- DOI: 10.1093/hmg/ddp373
Imprinting regulates mammalian snoRNA-encoding chromatin decondensation and neuronal nucleolar size
Abstract
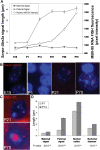
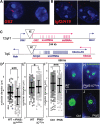
Imprinting, non-coding RNA and chromatin organization are modes of epigenetic regulation that modulate gene expression and are necessary for mammalian neurodevelopment. The only two known mammalian clusters of genes encoding small nucleolar RNAs (snoRNAs), SNRPN through UBE3A(15q11-q13/7qC) and GTL2(14q32.2/12qF1), are neuronally expressed, localized to imprinted loci and involved in at least five neurodevelopmental disorders. Deficiency of the paternal 15q11-q13 snoRNA HBII-85 locus is necessary to cause the neurodevelopmental disorder Prader-Willi syndrome (PWS). Here we show epigenetically regulated chromatin decondensation at snoRNA clusters in human and mouse brain. An 8-fold allele-specific decondensation of snoRNA chromatin was developmentally regulated specifically in maturing neurons, correlating with HBII-85 nucleolar accumulation and increased nucleolar size. Reciprocal mouse models revealed a genetic and epigenetic requirement of the 35 kb imprinting center (IC) at the Snrpn-Ube3a locus for transcriptionally regulated chromatin decondensation. PWS human brain and IC deletion mouse Purkinje neurons showed significantly decreased nucleolar size, demonstrating the essential role of the 15q11-q13 HBII-85 locus in neuronal nucleolar maturation. These results are relevant to understanding the molecular pathogenesis of multiple human neurodevelopmental disorders, including PWS and some causes of autism.
Figures


References
-
- Mehler M.F., Mattick J.S. Noncoding RNAs and RNA editing in brain development, functional diversification, and neurological disease. Physiol. Rev. 2007;87:799–823. - PubMed
-
- Wilkinson L.S., Davies W., Isles A.R. Genomic imprinting effects on brain development and function. Nat. Rev. Neurosci. 2007;8:832–843. - PubMed
-
- Mercer T.R., Dinger M.E., Mariani J., Kosik K.S., Mehler M.F., Mattick J.S. Noncoding RNAs in long-term memory formation. Neuroscientist. 2008;14:434–445. - PubMed
-
- Morison I.M., Ramsay J.P., Spencer H.G. A census of mammalian imprinting. Trends Genet. 2005;21:457–465. - PubMed
-
- Chambeyron S., Da Silva N.R., Lawson K.A., Bickmore W.A. Nuclear re-organisation of the Hoxb complex during mouse embryonic development. Development. 2005;132:2215–2223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

