Analysis of the Fc gamma receptor-dependent component of neutralization measured by anthrax toxin neutralization assays
- PMID: 19656993
- PMCID: PMC2756837
- DOI: 10.1128/CVI.00194-09
Analysis of the Fc gamma receptor-dependent component of neutralization measured by anthrax toxin neutralization assays
Abstract
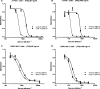
Anthrax toxin neutralization assays are used to measure functional antibody levels elicited by anthrax vaccines in both preclinical and clinical studies. In this study, we investigated the magnitude and molecular nature of Fc gamma (Fcgamma) receptor-dependent toxin neutralization observed in commonly used forms of the anthrax toxin neutralization assay. Significantly more Fcgamma receptor-dependent neutralization was observed in the J774A.1 cell-based assay than in the RAW 264.7 cell-based assay, a finding that could be due to the larger numbers of Fcgamma receptors that we found on J774A.1 cells by using flow cytometry. Thus, the extent to which Fcgamma receptor-dependent neutralization contributes to the total neutralization measured by the assay depends on the specific cell type utilized in the assay. Using Fcgamma receptor blocking monoclonal antibodies, we found that at least three murine Fcgamma receptor classes, IIB, III, and IV, can contribute to Fcgamma receptor-dependent neutralization. When antibodies elicited by immunization of rabbits with protective-antigen-based anthrax vaccines were analyzed, we found that the magnitude of Fcgamma receptor-dependent neutralization observed in the J774A.1 cell-based assay was dependent on the concentration of protective antigen utilized in the assay. Our results suggest that the characteristics of the antibodies analyzed in the assay (e.g., species of origin, isotype, and subclass), as well as the assay design (e.g., cell type and protective antigen concentration), could significantly influence the extent to which Fcgamma receptor-dependent neutralization contributes to the total neutralization measured by anthrax toxin neutralization assays. These findings should be considered when interpreting anthrax toxin neutralization assay output.
Figures
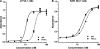

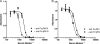
References
-
- Boyden, E. D., and W. F. Dietrich. 2006. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38:240-244. - PubMed
-
- Duesbery, N. S., C. P. Webb, S. H. Leppla, V. M. Gordon, K. R. Klimpel, T. D. Copeland, N. G. Ahn, M. K. Oskarsson, K. Fukasawa, K. D. Paull, and G. F. Vande Woude. 1998. Proteolytic inactivation of MAP-kinase-kinase by anthrax lethal factor. Science 280:734-737. - PubMed
-
- FDA. 2002. New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Fed. Regist. 67:37988-37998. - PubMed
-
- FDA. 2002. Anthrax vaccines: efficacy testing and surrogate markers of immunity workshop. http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/Workshops....
-
- FDA. 2007. Anthrax vaccines: bridging correlates of protection in animals to immunogenicity in humans. http://www.fda.gov/downloads/BiologicsBloodVaccines/NewsEvents/Workshops....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

