Towards a unified theory for morphomechanics
- PMID: 19657011
- PMCID: PMC2865877
- DOI: 10.1098/rsta.2009.0100
Towards a unified theory for morphomechanics
Abstract
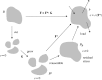
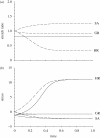
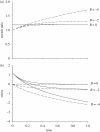
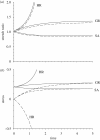
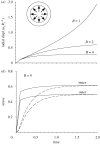
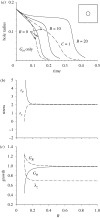
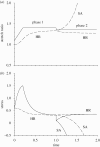
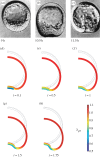
Mechanical forces are closely involved in the construction of an embryo. Experiments have suggested that mechanical feedback plays a role in regulating these forces, but the nature of this feedback is poorly understood. Here, we propose a general principle for the mechanics of morphogenesis, as governed by a pair of evolution equations based on feedback from tissue stress. In one equation, the rate of growth (or contraction) depends on the difference between the current tissue stress and a target (homeostatic) stress. In the other equation, the target stress changes at a rate that depends on the same stress difference. The parameters in these morphomechanical laws are assumed to depend on stress rate. Computational models are used to illustrate how these equations can capture a relatively wide range of behaviours observed in developing embryos, as well as show the limitations of this theory. Specific applications include growth of pressure vessels (e.g. the heart, arteries and brain), wound healing and sea urchin gastrulation. Understanding the fundamental principles of tissue construction can help engineers design new strategies for creating replacement tissues and organs in vitro.
Figures


References
-
- Alberch P. 1980. Ontogenesis and morphological diversification. Am. Zool. 20, 653–667.
-
- Ambrosi D., Guana F. 2007. Stress-modulated growth. Math. Mech. Solids 12, 319–342. (10.1177/1081286505059739) - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
