Stability of electrical coupling despite massive developmental changes of intrinsic neuronal physiology
- PMID: 19657029
- PMCID: PMC3353772
- DOI: 10.1523/JNEUROSCI.4568-08.2009
Stability of electrical coupling despite massive developmental changes of intrinsic neuronal physiology
Abstract
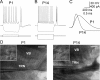
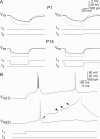
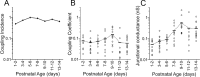
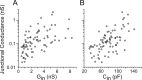
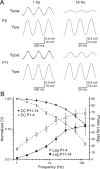
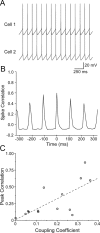
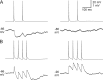
Gap junctions mediate metabolic and electrical interactions between some cells of the CNS. For many types of neurons, gap junction-mediated electrical coupling is most prevalent during early development, then decreases sharply with maturation. However, neurons in the thalamic reticular nucleus (TRN), which exert powerful inhibitory control over thalamic relay cells, are electrically coupled in relatively mature animals. It is not known whether TRN cells or any neurons that are electrically coupled when mature are also coupled during early development. We used dual whole-cell recordings in mouse brain slices to study the postnatal development of electrical and chemical synapses that interconnect TRN neurons. Inhibitory chemical synapses were seen as early as postnatal day 4 but were infrequent at all ages, whereas TRN cells were extensively connected by electrical synapses from birth onward. Surprisingly, the functional strength of electrical coupling, assayed under steady-state conditions or during spiking, remained relatively constant as the brain matured despite dramatic concurrent changes of intrinsic membrane properties. Most notably, neuronal input resistances declined almost eightfold during the first two postnatal weeks, but there were offsetting increases in gap junctional conductances. This suggests that the size or number of gap junctions increase homeostatically to compensate for leakier nonjunctional membranes. Additionally, we found that the ability of electrical synapses to synchronize high frequency subthreshold signals improved as TRN cells matured. Our results demonstrate that certain central neurons may maintain or even increase their gap junctional communication as they mature.
Figures


Similar articles
-
Electrical synapses and the development of inhibitory circuits in the thalamus.J Physiol. 2016 May 15;594(10):2579-92. doi: 10.1113/JP271880. Epub 2016 Mar 23. J Physiol. 2016. PMID: 26864476 Free PMC article.
-
Dopamine receptors D1, D2, and D4 modulate electrical synapses and excitability in the thalamic reticular nucleus.J Neurophysiol. 2025 Feb 1;133(2):374-387. doi: 10.1152/jn.00260.2024. Epub 2024 Dec 20. J Neurophysiol. 2025. PMID: 39706150 Free PMC article.
-
Electrical and chemical synapses between relay neurons in developing thalamus.J Physiol. 2010 Jul 1;588(Pt 13):2403-15. doi: 10.1113/jphysiol.2010.187096. Epub 2010 May 10. J Physiol. 2010. PMID: 20457735 Free PMC article.
-
Connexon connexions in the thalamocortical system.Prog Brain Res. 2005;149:41-57. doi: 10.1016/S0079-6123(05)49004-4. Prog Brain Res. 2005. PMID: 16226575 Review.
-
Bursts modify electrical synaptic strength.Brain Res. 2012 Dec 3;1487:140-9. doi: 10.1016/j.brainres.2012.05.061. Epub 2012 Jul 5. Brain Res. 2012. PMID: 22771703 Free PMC article. Review.
Cited by
-
Characteristics and plasticity of electrical synaptic transmission.BMC Cell Biol. 2016 May 24;17 Suppl 1(Suppl 1):13. doi: 10.1186/s12860-016-0091-y. BMC Cell Biol. 2016. PMID: 27230893 Free PMC article. Review.
-
Heterozygous expression of a Kcnt1 gain-of-function variant has differential effects on SST- and PV-expressing cortical GABAergic neurons.bioRxiv [Preprint]. 2024 Aug 2:2023.10.11.561953. doi: 10.1101/2023.10.11.561953. bioRxiv. 2024. Update in: Elife. 2024 Oct 11;13:RP92915. doi: 10.7554/eLife.92915. PMID: 37873369 Free PMC article. Updated. Preprint.
-
Synergistic plasticity of intrinsic conductance and electrical coupling restores synchrony in an intact motor network.Elife. 2016 Aug 23;5:e16879. doi: 10.7554/eLife.16879. Elife. 2016. PMID: 27552052 Free PMC article.
-
Heterozygous expression of a Kcnt1 gain-of-function variant has differential effects on somatostatin- and parvalbumin-expressing cortical GABAergic neurons.Elife. 2024 Oct 11;13:RP92915. doi: 10.7554/eLife.92915. Elife. 2024. PMID: 39392867 Free PMC article.
-
Functionally Distinct Circuits Are Linked by Heterocellular Electrical Synapses in the Thalamic Reticular Nucleus.eNeuro. 2024 Jan 10;11(1):ENEURO.0269-23.2023. doi: 10.1523/ENEURO.0269-23.2023. Print 2024 Jan. eNeuro. 2024. PMID: 38164593 Free PMC article.
References
-
- Agmon A, Connors BW. Thalamocortical responses of mouse somatosensory (barrel) cortex in vitro. Neuroscience. 1991;41:365–379. - PubMed
-
- Altman J, Bayer SA. Development of the rat thalamus: III. Time and site of origin and settling pattern of neurons of the reticular nucleus. J Comp Neurol. 1988;275:406–428. - PubMed
-
- Arumugam H, Liu X, Colombo PJ, Corriveau RA, Belousov AB. NMDA receptors regulate developmental gap junction uncoupling via CREB signaling. Nat Neurosci. 2005;8:1720–1726. - PubMed
-
- Bennett MV. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966;137:509–539. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
