Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons
- PMID: 19657032
- PMCID: PMC2763510
- DOI: 10.1523/JNEUROSCI.1544-09.2009
Transient receptor potential canonical 5 channels activate Ca2+/calmodulin kinase Igamma to promote axon formation in hippocampal neurons
Abstract
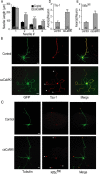
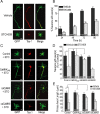
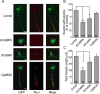
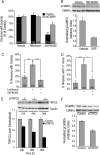
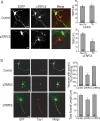
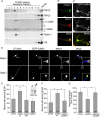
Functionality of neurons is dependent on their compartmentalized polarization of dendrites and an axon. The rapid and selective outgrowth of one neurite, relative to the others, to form the axon is critical in initiating neuronal polarity. Axonogenesis is regulated in part by an optimal intracellular calcium concentration. Our investigation of Ca(2+)-signaling pathways involved in axon formation using cultured hippocampal neurons demonstrates a role for Ca(2+)/calmodulin kinase kinase (CaMKK) and its downstream target Ca(2+)/calmodulin kinase I (CaMKI). Expression of constitutively active CaMKI induced formation of multiple axons, whereas blocking CaMKK or CaMKI activity with pharmacological, dominant-negative, or short hairpin RNA (shRNA) methods significantly inhibited axon formation. CaMKK signals via the gamma-isoform of CaMKI as shRNA to CaMKIgamma, but not the other CaMKI isoforms, inhibited axon formation. Furthermore, overexpression of wild-type CaMKIgamma, but not a mutant incapable of membrane association, accelerated the rate of axon formation. Pharmacological or small interfering RNA inhibition of transient receptor potential canonical 5 (TRPC5) channels, which are present in developing axonal growth cones, suppressed CaMKK-mediated activation of CaMKIgamma as well as axon formation. We demonstrate using biochemical fractionation and immunocytochemistry that CaMKIgamma and TRPC5 colocalize to lipid rafts. These results are consistent with a model in which highly localized calcium influx through the TRPC5 channels activates CaMKK and CaMKIgamma, which subsequently promote axon formation.
Figures


Comment in
-
CaMKK-CaMKI signaling pathways differentially control axon and dendrite elongation in cortical neurons.J Neurosci. 2010 Feb 24;30(8):2807-9. doi: 10.1523/JNEUROSCI.5984-09.2010. J Neurosci. 2010. PMID: 20181577 Free PMC article. Review. No abstract available.
References
-
- Alfonso S, Benito O, Alicia S, Angélica Z, Patricia G, Diana K, Vaca L, Luis V. Regulation of the cellular localization and function of human transient receptor potential channel 1 by other members of the TRPC family. Cell Calcium. 2008;43:375–387. - PubMed
-
- Alicia S, Angélica Z, Carlos S, Alfonso S, Vaca L. STIM1 converts TRPC1 from a receptor-operated to a store-operated channel: moving TRPC1 in and out of lipid rafts. Cell Calcium. 2008;44:479–491. - PubMed
-
- Ambudkar IS. Ca2+ signaling microdomains: platforms for the assembly and regulation of TRPC channels. Trends Pharmacol Sci. 2006;27:25–32. - PubMed
-
- Ambudkar IS, Ong HL. Organization and function of TRPC channelosomes. Pflugers Arch. 2007;455:187–200. - PubMed
-
- Ambudkar IS, Bandyopadhyay BC, Liu X, Lockwich TP, Paria B, Ong HL. Functional organization of TRPC-Ca2+ channels and regulation of calcium microdomains. Cell Calcium. 2006;40:495–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
