Epac increases melanoma cell migration by a heparan sulfate-related mechanism
- PMID: 19657062
- PMCID: PMC2770742
- DOI: 10.1152/ajpcell.00129.2009
Epac increases melanoma cell migration by a heparan sulfate-related mechanism
Abstract
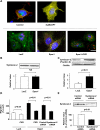
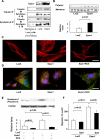
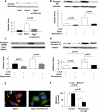
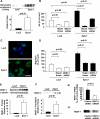
Melanoma, the most malignant form of human skin cancer, has a poor prognosis due to its strong metastatic ability. It was recently demonstrated that Epac, an effector molecule of cAMP, is involved in regulating cell migration; however, the role of Epac in melanoma cell migration remains unclear. We thus examined whether Epac regulates cell migration and metastasis of melanoma. Epac activation, by either specific agonist or overexpression of Epac, increased melanoma cell migration. Deletion of endogenous Epac with small interfering RNA decreased basal melanoma cell migration. These data suggested a major role of Epac in melanoma cell migration. Epac-induced cell migration was mediated by translocation of syndecan-2, a cell-surface heparan sulfate proteoglycan, to lipid rafts. This syndecan-2 translocation was regulated by tubulin polymerization via the Epac/phosphoinositol-3 kinase pathway. Epac-induced cell migration was also regulated by the production of heparan sulfate, a major extracellular matrix. Epac-induced heparan sulfate production was attributable to the increased expression of N-deacetylase/N-sulfotransferase-1 (NDST-1) accompanied by an increased NDST-1 translation rate. Finally, Epac overexpression enhanced lung colonization of melanoma cells in mice. Taken together, these data indicate that Epac regulates melanoma cell migration/metastasis mostly via syndecan-2 translocation and heparan sulfate production.
Figures


Comment in
-
Epac in melanoma: a contributor to tumor cell physiology? Focus on "Epac increases melanoma cell migration by a heparin sulfate-related mechanism".Am J Physiol Cell Physiol. 2009 Oct;297(4):C797-9. doi: 10.1152/ajpcell.00358.2009. Epub 2009 Aug 19. Am J Physiol Cell Physiol. 2009. PMID: 19692651 No abstract available.
References
-
- Benoist F and Grand-Perret T. Cotranslational degradation of apolipoprotein B100 by the proteasome is prevented by microsomal triglyceride transfer protein synchronized translation studies on HepG2 cells treated with an inhibitor of microsomal triglyceride transfer protein. J Biol Chem 272: 20435–20442, 1997 - PubMed
-
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Ann Rev Biochem 68: 729–777, 1999 - PubMed
-
- Berwick M, Wiggins C. The current epidemiology of cutaneous malignant melanoma. Front Biosci 11: 1244–1254, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

