Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton
- PMID: 19657144
- PMCID: PMC2785673
- DOI: 10.1074/jbc.M109.023572
Increased lipid oxidation causes oxidative stress, increased peroxisome proliferator-activated receptor-gamma expression, and diminished pro-osteogenic Wnt signaling in the skeleton
Abstract
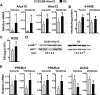
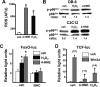
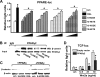
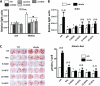
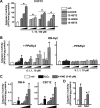
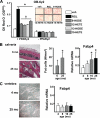
Loss of bone mass with advancing age in mice is because of decreased osteoblast number and is associated with increased oxidative stress and decreased canonical Wnt signaling. However, the underlying mechanisms are poorly understood. We report an age-related increase in the lipid oxidation product 4-hydroxynonenal (4-HNE) as well as increased expression of lipoxygenase and peroxisome proliferator-activated receptor-gamma (PPARgamma) in the murine skeleton. These changes together with decreased Wnt signaling are reproduced in 4-month-old mice bearing a high expressing allele of the lipoxygenase Alox15. The addition of 4-HNE to cultured osteoblastic cells increases oxidative stress, which in turn diverts beta-catenin from T-cell-specific transcription factors to Forkhead box O (FoxO) transcription factors, thereby attenuating the suppressive effect of beta-catenin on PPARgamma gene expression. Oxidized lipids, acting as ligands of PPARgamma, promote binding of PPARgamma2 to beta-catenin and reduce the levels of the latter, and they attenuate Wnt3a-stimulated proliferation and osteoblast differentiation. Furthermore, oxidized lipids and 4-HNE stimulate apoptosis of osteoblastic cells. In view of the role of oxidized lipids in atherogenesis, the adverse effects of lipoxygenase-mediated lipid oxidation on the differentiation and survival of osteoblasts may provide a mechanistic explanation for the link between atherosclerosis and osteoporosis.
Figures
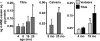

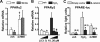

References
-
- Lips P., Courpron P., Meunier P. J. (1978) Calcif. Tissue Res. 26, 13–17 - PubMed
-
- Parfitt A. M. (1990) in Bone: Volume 1. The Osteoblast and Osteocyte (Hall B. K. ed) pp. 351–429, Telford Press and CRC Press, Boca Raton, FL
-
- Tyner S. D., Venkatachalam S., Choi J., Jones S., Ghebranious N., Igelmann H., Lu X., Soron G., Cooper B., Brayton C., Hee Park S., Thompson T., Karsenty G., Bradley A., Donehower L. A. (2002) Nature 415, 45–53 - PubMed
-
- Jilka R. L. (2002) BoneKey-Osteovision 10.1138/2002024 - DOI
-
- Ali A. A., Weinstein R. S., Stewart S. A., Parfitt A. M., Manolagas S. C., Jilka R. L. (2005) Endocrinology 146, 1226–1235 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

