The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy
- PMID: 19657325
- PMCID: PMC2963106
- DOI: 10.1038/ki.2009.296
The role of VHL in clear-cell renal cell carcinoma and its relation to targeted therapy
Abstract
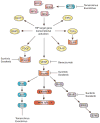
The basic biology underlying the development of clear-cell renal cell carcinoma (ccRCC) is critically dependent on the von Hippel-Lindau gene (VHL), whose protein product is important in the cell's normal response to hypoxia. Aberrations in VHL's function, either through mutation or promoter hypermethylation, lead to accumulation of the transcriptional regulatory molecule, hypoxia-inducible factor alpha (HIFalpha). HIFalpha can then dimerize with HIFbeta and translocate to the nucleus, where it will transcriptionally upregulate a series of hypoxia-responsive genes, including vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and others. Binding of these ligands to their cognate receptors activates a series of kinase- dependent signaling pathways, including the RAF-MEK-ERK and phosphatidylinositol-3 kinase-AKT-mTOR pathways. Targeted agents developed and now approved for use in advanced ccRCC include humanized monoclonal antibodies against VEGF, small-molecule tyrosine kinase inhibitors, and inhibitors of mTOR. Understanding the biology of ccRCC is critical in understanding the current therapy for the disease and in developing novel therapeutics in the future. This review will provide an overview of the genetics of ccRCC, with an emphasis on how this has informed the development of the targeted therapeutics for this disease.
Conflict of interest statement
The author declared no competing interests.
Figures

References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol. 2001;19:148–154. - PubMed
-
- Lonser RR, Glenn GM, Walther M, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–2067. - PubMed
-
- Grubb RL, III, Choyke PL, Pinto PA, et al. Management of von Hippel-Lindau-associated kidney cancer. Nat Clin Pract Urol. 2005;2:248–255. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

