Developmental and species-divergent globin switching are driven by BCL11A
- PMID: 19657335
- PMCID: PMC3749913
- DOI: 10.1038/nature08243
Developmental and species-divergent globin switching are driven by BCL11A
Abstract
The contribution of changes in cis-regulatory elements or trans-acting factors to interspecies differences in gene expression is not well understood. The mammalian beta-globin loci have served as a model for gene regulation during development. Transgenic mice containing the human beta-globin locus, consisting of the linked embryonic (epsilon), fetal (gamma) and adult (beta) genes, have been used as a system to investigate the temporal switch from fetal to adult haemoglobin, as occurs in humans. Here we show that the human gamma-globin (HBG) genes in these mice behave as murine embryonic globin genes, revealing a limitation of the model and demonstrating that critical differences in the trans-acting milieu have arisen during mammalian evolution. We show that the expression of BCL11A, a repressor of human gamma-globin expression identified by genome-wide association studies, differs between mouse and human. Developmental silencing of the mouse embryonic globin and human gamma-globin genes fails to occur in mice in the absence of BCL11A. Thus, BCL11A is a critical mediator of species-divergent globin switching. By comparing the ontogeny of beta-globin gene regulation in mice and humans, we have shown that alterations in the expression of a trans-acting factor constitute a critical driver of gene expression changes during evolution.
Conflict of interest statement
The authors declare competing financial interests: V.G.S. and S.H.O. are inventors on a patent filed by the Children’s Hospital of Boston related to the therapeutic targeting of BCL11A for induction of fetal hemoglobin in humans.
Figures
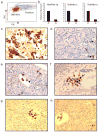
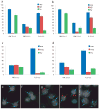


References
-
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. - PubMed
-
- Hoekstra HE, Coyne JA. The locus of evolution: evo devo and the genetics of adaptation. Evolution. 2007;61:995–1016. - PubMed
-
- Wallace HA, et al. Manipulating the mouse genome to engineer precise functional syntenic replacements with human sequence. Cell. 2007;128:197–209. - PubMed
-
- McGrath K, Palis J. Ontogeny of erythropoiesis in the mammalian embryo. Curr Top Dev Biol. 2008;82:1–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

