SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development
- PMID: 19657336
- PMCID: PMC2903203
- DOI: 10.1038/nn.2387
SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development
Abstract
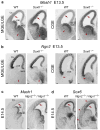
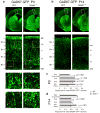
The neuronal diversity of the CNS emerges largely from controlled spatial and temporal segregation of cell type-specific molecular regulators. We found that the transcription factor SOX6 controls the molecular segregation of dorsal (pallial) from ventral (subpallial) telencephalic progenitors and the differentiation of cortical interneurons, regulating forebrain progenitor and interneuron heterogeneity. During corticogenesis in mice, SOX6 and SOX5 were largely mutually exclusively expressed in pallial and subpallial progenitors, respectively, and remained mutually exclusive in a reverse pattern in postmitotic neuronal progeny. Loss of SOX6 from pallial progenitors caused their inappropriate expression of normally subpallium-restricted developmental controls, conferring mixed dorsal-ventral identity. In postmitotic cortical interneurons, loss of SOX6 disrupted the differentiation and diversity of cortical interneuron subtypes, analogous to SOX5 control over cortical projection neuron development. These data indicate that SOX6 is a central regulator of both progenitor and cortical interneuron diversity during neocortical development.
Figures






References
-
- Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. - PubMed
-
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. - PubMed
-
- Molyneaux BJ, Arlotta P, Menezes JR, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

