Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes
- PMID: 19657368
- PMCID: PMC2777742
- DOI: 10.1038/leu.2009.158
Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes
Abstract
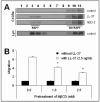
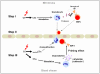
We reported that complement cascade (CC) becomes activated in bone marrow (BM) during granulocyte colony-stimulating factor (G-CSF) mobilization of hematopoietic stem/progenitor cells (HSPCs) and showed that, although third CC component (C3)-deficient mice are easy mobilizers, fifth CC component (C5)-deficient mice mobilize very poorly. To explain this, we postulated that activation/cleavage of CC releases C3a and C5a anaphylatoxins that differently regulate mobilization. Accordingly, C3a, by enhancing responsiveness of HSPCs to decreasing concentrations of stromal-derived growth factor-1 (SDF-1) in BM, prevents mobilization and promotes their BM retention. Therefore, in this study, we focused on the mobilization-enhancing role of C5a. We found that C5a receptor (C5aR) is not expressed on the surface of HSPCs, and that C5a-mediated promobilization effects are mediated by stimulation of granulocytes. Overall, our data support the following model. First C5aR(+) granulocytes are chemoattracted by plasma C5 cleavage fragments, being the first wave of cells leaving BM. This facilitates a subsequent egress of HSPCs. In the next step, after leaving BM, granulocytes undergo degranulation in response to plasma C5a and secrete some cationic peptides (cathelicidin, beta-defensin) that, as shown here for the first time, highly enhance the responsiveness of HSPCs to plasma SDF-1 gradient. In conclusion, our data reveal the underappreciated central role of innate immunity in mobilization, in which C5 cleavage fragments through granulocytes orchestrate this process.
Figures

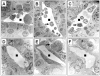
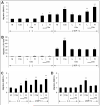
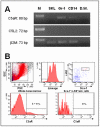
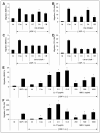
Similar articles
-
Fifth complement cascade protein (C5) cleavage fragments disrupt the SDF-1/CXCR4 axis: further evidence that innate immunity orchestrates the mobilization of hematopoietic stem/progenitor cells.Exp Hematol. 2010 Apr;38(4):321-32. doi: 10.1016/j.exphem.2010.02.002. Epub 2010 Feb 12. Exp Hematol. 2010. PMID: 20153802 Free PMC article.
-
Signaling of the Complement Cleavage Product Anaphylatoxin C5a Through C5aR (CD88) Contributes to Pharmacological Hematopoietic Stem Cell Mobilization.Stem Cell Rev Rep. 2017 Dec;13(6):793-800. doi: 10.1007/s12015-017-9769-6. Stem Cell Rev Rep. 2017. PMID: 28918528 Free PMC article.
-
Evidence that a lipolytic enzyme--hematopoietic-specific phospholipase C-β2--promotes mobilization of hematopoietic stem cells by decreasing their lipid raft-mediated bone marrow retention and increasing the promobilizing effects of granulocytes.Leukemia. 2016 Apr;30(4):919-28. doi: 10.1038/leu.2015.315. Epub 2015 Nov 19. Leukemia. 2016. PMID: 26582648 Free PMC article.
-
Innate immunity: a key player in the mobilization of hematopoietic stem/progenitor cells.Arch Immunol Ther Exp (Warsz). 2009 Jul-Aug;57(4):269-78. doi: 10.1007/s00005-009-0037-6. Epub 2009 Jul 4. Arch Immunol Ther Exp (Warsz). 2009. PMID: 19578812 Review.
-
A pivotal role of activation of complement cascade (CC) in mobilization of hematopoietic stem/progenitor cells (HSPC).Adv Exp Med Biol. 2008;632:47-60. Adv Exp Med Biol. 2008. PMID: 19025113 Review.
Cited by
-
MT1-MMP and RECK: opposite and essential roles in hematopoietic stem and progenitor cell retention and migration.J Mol Med (Berl). 2011 Dec;89(12):1167-74. doi: 10.1007/s00109-011-0792-9. Epub 2011 Aug 13. J Mol Med (Berl). 2011. PMID: 21842347 Review.
-
The Emerging Link Between the Complement Cascade and Purinergic Signaling in Stress Hematopoiesis.Front Immunol. 2018 Jun 5;9:1295. doi: 10.3389/fimmu.2018.01295. eCollection 2018. Front Immunol. 2018. PMID: 29922299 Free PMC article. Review.
-
Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex.Leukemia. 2010 May;24(5):976-85. doi: 10.1038/leu.2010.53. Epub 2010 Apr 1. Leukemia. 2010. PMID: 20357827 Free PMC article.
-
Novel evidence that the mannan-binding lectin pathway of complement activation plays a pivotal role in triggering mobilization of hematopoietic stem/progenitor cells by activation of both the complement and coagulation cascades.Leukemia. 2017 Jan;31(1):262-265. doi: 10.1038/leu.2016.278. Epub 2016 Oct 13. Leukemia. 2017. PMID: 27733776 Free PMC article. No abstract available.
-
Markers of Regenerative Processes in Patients with Bipolar Disorder: A Case-control Study.Brain Sci. 2020 Jun 30;10(7):408. doi: 10.3390/brainsci10070408. Brain Sci. 2020. PMID: 32629800 Free PMC article.
References
-
- Kyne L, Hausdorff JM, Knight E, Dukas L, Azhar G, Wei JY. Neutrophilia and congestive heart failure after acute myocardial infarction. Am Heart J. 2000;139:94–100. - PubMed
-
- Matsunaga T, Sakamaki S, Kohgo Y, Ohi S, Hirayama Y, Niitsu Y. Recombinant human granulocyte colony-stimulating factor can mobilize sufficient amounts of peripheral blood stem cells in healthy volunteers for allogeneic transplantation. Bone Marrow Transplant. 1993;11:103–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

