Inflammation and proliferation act together to mediate intestinal cell fusion
- PMID: 19657387
- PMCID: PMC2716548
- DOI: 10.1371/journal.pone.0006530
Inflammation and proliferation act together to mediate intestinal cell fusion
Abstract
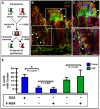
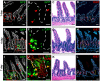
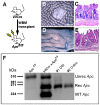
Cell fusion between circulating bone marrow-derived cells (BMDCs) and non-hematopoietic cells is well documented in various tissues and has recently been suggested to occur in response to injury. Here we illustrate that inflammation within the intestine enhanced the level of BMDC fusion with intestinal progenitors. To identify important microenvironmental factors mediating intestinal epithelial cell fusion, we performed bone marrow transplantation into mouse models of inflammation and stimulated epithelial proliferation. Interestingly, in a non-injury model or in instances where inflammation was suppressed, an appreciable baseline level of fusion persisted. This suggests that additional mediators of cell fusion exist. A rigorous temporal analysis of early post-transplantation cellular dynamics revealed that GFP-expressing donor cells first trafficked to the intestine coincident with a striking increase in epithelial proliferation, advocating for a required fusogenic state of the host partner. Directly supporting this hypothesis, induction of augmented epithelial proliferation resulted in a significant increase in intestinal cell fusion. Here we report that intestinal inflammation and epithelial proliferation act together to promote cell fusion. While the physiologic impact of cell fusion is not yet known, the increased incidence in an inflammatory and proliferative microenvironment suggests a potential role for cell fusion in mediating the progression of intestinal inflammatory diseases and cancer.
Conflict of interest statement
Figures


References
-
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. - PubMed
-
- Camargo FD, Green R, Capetanaki Y, Jackson KA, Goodell MA. Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nat Med. 2003;9:1520–1527. - PubMed
-
- Corbel SY, Lee A, Yi L, Duenas J, Brazelton TR, et al. Contribution of hematopoietic stem cells to skeletal muscle. Nat Med. 2003;9:1528–1532. - PubMed
-
- Ferrari G, Cusella-De Angelis G, Coletta M, Paolucci E, Stornaiuolo A, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

