Stem-like ovarian cancer cells can serve as tumor vascular progenitors
- PMID: 19658191
- PMCID: PMC2783765
- DOI: 10.1002/stem.191
Stem-like ovarian cancer cells can serve as tumor vascular progenitors
Abstract
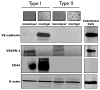
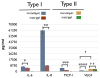
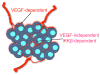
Neovascularization is required for solid tumor maintenance, progression, and metastasis. The most described contribution of cancer cells in tumor neovascularization is the secretion of factors, which attract various cell types to establish a microenvironment that promotes blood vessel formation. The cancer stem cell hypothesis suggests that tumors are composed of cells that may share the differentiation capacity of normal stem cells. Similar to normal stem cells, cancer stem cells (CSCs) have the capacity to acquire different phenotypes. Thus, it is possible that CSCs have a bigger role in the process of tumor neovascularization. In this study, we show the capacity of a specific population of ovarian cancer cells with stem-like properties to give rise to xenograft tumors containing blood vessels, which are lined by human CD34+ cells. In addition, when cultured in high-density Matrigel, these cells mimic the behavior of normal endothelial cells and can form vessel-like structures in 24 hours. Microscopic analysis showed extensive branching and maturation of vessel-like structures in 7 days. Western blot and flow cytometry analysis showed that this process is accompanied by the acquisition of classic endothelial markers, CD34 and VE-cadherin. More importantly, we show that this process is vascular endothelial growth factor-independent, but IKK beta-dependent. Our findings suggest that anti-angiogenic therapies should take into consideration the inherent capacity of these cells to serve as vascular progenitors.
Figures







References
-
- Sato Y. Molecular diagnosis of tumor angiogenesis and anti-angiogenic cancer therapy. Int J Clin Oncol. 2003;8:200–206. - PubMed
-
- Folkman J, Shing Y. Angiogenesis. J Biol Chem. 1992;267:10931–10934. - PubMed
-
- Goh PP, Sze DM, Roufogalis BD. Molecular and cellular regulators of cancer angiogenesis. Curr Cancer Drug Targets. 2007;7:743–758. - PubMed
-
- Cao Y. Tumor angiogenesis and molecular targets for therapy. Front Biosci. 2009;14:3962–3973. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

