NRG1 / ERBB3 signaling in melanocyte development and melanoma: inhibition of differentiation and promotion of proliferation
- PMID: 19659570
- PMCID: PMC3023175
- DOI: 10.1111/j.1755-148X.2009.00616.x
NRG1 / ERBB3 signaling in melanocyte development and melanoma: inhibition of differentiation and promotion of proliferation
Abstract
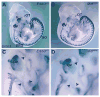
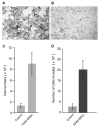
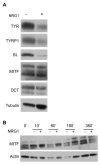
Neuregulin (NRG) signaling through the receptor tyrosine kinase, ERBB3, is required for embryonic development, and dysregulated signaling has been associated with cancer progression. Here, we show that NRG1/ERBB3 signaling inhibits melanocyte (MC) maturation and promotes undifferentiated, migratory and proliferative cellular characteristics. Embryonic analyses demonstrated that initial MC specification and distribution were not dependent on ERBB3 signaling. However NRG1/ERBB3 signaling was both necessary and sufficient to inhibit differentiation of later stages of MC development in culture. Analysis of tissue arrays of human melanoma samples suggests that ERBB3 signaling may also contribute to metastatic progression of melanoma as ERBB3 was phosphorylated in primary tumors compared with nevi or metastatic lesions. Neuregulin 1-treated MCs demonstrated increased proliferation and invasion and altered morphology concomitant with decreased levels of differentiation genes, increased levels of proliferation genes and altered levels of melanoma progression and metastases genes. ERBB3 activation in primary melanomas suggests that NRG1/ERBB3 signaling may contribute to the progression of melanoma from benign nevi to malignancies. We propose that targeting ERBB3 activation and downstream genes identified in this study may provide novel therapeutic interventions for malignant melanoma.
Figures


References
-
- Baxter LL, Hsu BJ, Umayam L, Wolfsberg TG, Larson DM, Frith MC, Kawai J, Hayashizaki Y, Carninci P, Pavan WJ. Informatic and genomic analysis of melanocyte cDNA libraries as a resource for the study of melanocyte development and function. Pigment Cell Res. 2007;20:201–9. - PubMed
-
- Baxter LL, Pavan WJ. Pmel17 expression is Mitf-dependent and reveals cranial melanoblast migration during murine development. Gene Expr Patterns. 2003;3:703–7. - PubMed
-
- Bennett DC. Colour genes, oncogenes and melanocyte differentiation. J Cell Sci. 1991;98(Pt 2):135–9. - PubMed
-
- Bennett DC. How to make a melanoma: what do we know of the primary clonal events? Pigment Cell Melanoma Res. 2008;21:27–38. - PubMed
-
- Bennett DC, Trayner ID, Piao X, Easty DJ, Kluppel M, Alexander WS, Wagner EF, Bernstein A. recessive spotting: a linked locus that interacts with W/Kit but is not allelic. Genes Cells. 1998;3:235–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

