A serum amyloid P-binding hydrogel speeds healing of partial thickness wounds in pigs
- PMID: 19660048
- PMCID: PMC2850269
- DOI: 10.1111/j.1524-475X.2009.00482.x
A serum amyloid P-binding hydrogel speeds healing of partial thickness wounds in pigs
Abstract
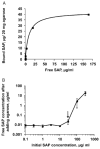
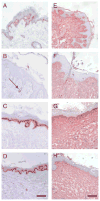
During wound healing, some circulating monocytes enter the wound, differentiate into fibroblast-like cells called fibrocytes, and appear to then further differentiate into myofibroblasts, cells that play a key role in collagen deposition, cytokine release, and wound contraction. The differentiation of monocytes into fibrocytes is inhibited by the serum protein serum amyloid P (SAP). Depleting SAP at a wound site thus might speed wound healing. SAP binds to some types of agarose in the presence of Ca(2+). We found that human SAP binds to an agarose with a K(D) of 7 x 10(-8) M and a B(max) of 2.1 microg SAP/mg wet weight agarose. Mixing this agarose 1 : 5 w/v with 30 microg/mL human SAP (the average SAP concentration in normal serum) in a buffer containing 2 mM Ca(2+) reduced the free SAP concentration to approximately 0.02 microg/mL, well below the concentration that inhibits fibrocyte differentiation. Compared with a hydrogel dressing and a foam dressing, dressings containing this agarose and Ca(2+) significantly increased the speed of wound healing in partial thickness wounds in pigs. This suggests that agarose/Ca(2+) dressings may be beneficial for wound healing in humans.
Figures


Similar articles
-
The Development of Serum Amyloid P as a Possible Therapeutic.Front Immunol. 2018 Oct 16;9:2328. doi: 10.3389/fimmu.2018.02328. eCollection 2018. Front Immunol. 2018. PMID: 30459752 Free PMC article. Review.
-
Serum amyloid P inhibits dermal wound healing.Wound Repair Regen. 2008 Mar-Apr;16(2):266-73. doi: 10.1111/j.1524-475X.2008.00366.x. Wound Repair Regen. 2008. PMID: 18318811 Free PMC article.
-
Engineering Bioactive Self-Healing Antibacterial Exosomes Hydrogel for Promoting Chronic Diabetic Wound Healing and Complete Skin Regeneration.Theranostics. 2019 Jan 1;9(1):65-76. doi: 10.7150/thno.29766. eCollection 2019. Theranostics. 2019. PMID: 30662554 Free PMC article.
-
The impact of first-aid dressing design on healing of porcine partial thickness wounds.Wound Repair Regen. 2019 Nov;27(6):622-633. doi: 10.1111/wrr.12747. Epub 2019 Jul 23. Wound Repair Regen. 2019. PMID: 31276609
-
Topical metformin in wound healing: a comprehensive systematic review of therapeutic outcomes.Arch Dermatol Res. 2025 May 16;317(1):760. doi: 10.1007/s00403-025-04277-w. Arch Dermatol Res. 2025. PMID: 40377758 Review.
Cited by
-
Differentiation of circulating monocytes into fibroblast-like cells.Methods Mol Biol. 2012;904:191-206. doi: 10.1007/978-1-61779-943-3_16. Methods Mol Biol. 2012. PMID: 22890933 Free PMC article.
-
The Development of Serum Amyloid P as a Possible Therapeutic.Front Immunol. 2018 Oct 16;9:2328. doi: 10.3389/fimmu.2018.02328. eCollection 2018. Front Immunol. 2018. PMID: 30459752 Free PMC article. Review.
-
A brief exposure to tryptase or thrombin potentiates fibrocyte differentiation in the presence of serum or serum amyloid p.J Immunol. 2015 Jan 1;194(1):142-50. doi: 10.4049/jimmunol.1401777. Epub 2014 Nov 26. J Immunol. 2015. PMID: 25429068 Free PMC article.
-
Brain serum amyloid P levels are reduced in individuals that lack dementia while having Alzheimer's disease neuropathology.Neurochem Res. 2012 Apr;37(4):795-801. doi: 10.1007/s11064-011-0674-0. Epub 2011 Dec 29. Neurochem Res. 2012. PMID: 22205573 Free PMC article.
-
Cell density sensing and size determination.Dev Growth Differ. 2011 May;53(4):482-94. doi: 10.1111/j.1440-169X.2010.01248.x. Epub 2011 Apr 27. Dev Growth Differ. 2011. PMID: 21521184 Free PMC article. Review.
References
-
- Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol. 2004;36:598–606. - PubMed
-
- Postlethwaite AE, Shigemitsu H, Kanangat S. Cellular origins of fibroblasts: possible implications for organ fibrosis in systemic sclerosis. Curr Opin Rheumatol. 2004;16:733–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

