A review of COFRADIC techniques targeting protein N-terminal acetylation
- PMID: 19660099
- PMCID: PMC2722099
- DOI: 10.1186/1753-6561-3-S6-S6
A review of COFRADIC techniques targeting protein N-terminal acetylation
Abstract
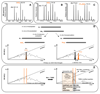
Acetylation of nascent protein Nalpha-termini is a common modification among archae and eukaryotes and can influence the structure and function of target proteins. This modification has been studied on an individual protein or (synthetic) peptide level or on a proteome scale using two-dimensional polyacrylamide gel electrophoresis. We recently developed mass spectrometry driven proteome analytical approaches specifically targeting the amino (N) terminus of proteins based on the concept of diagonal reverse-phase chromatography. We here review how this so-called combined fractional diagonal chromatography (COFRADIC) technique can be used in combination with differential mass-tagging strategies as to both qualitatively and quantitatively assess protein Nalpha-acetylation in whole proteomes.
Figures

References
-
- Kawasaki H, Imajoh S, Suzuki K. Separation of peptides on the basis of the difference in positive charge: simultaneous isolation of C-terminal and blocked N-terminal peptides from tryptic digests. J Biochem (Tokyo) 1987;102:393–400. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

