Probing binding determinants in center P of the cytochrome bc(1) complex using novel hydroxy-naphthoquinones
- PMID: 19660431
- PMCID: PMC2787711
- DOI: 10.1016/j.bbabio.2009.07.010
Probing binding determinants in center P of the cytochrome bc(1) complex using novel hydroxy-naphthoquinones
Abstract
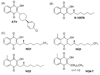
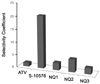
Atovaquone is a substituted 2-hydroxy-naphthoquinone used therapeutically against Plasmodium falciparum (malaria) and Pneumocystis pathogens. It acts by inhibiting the cytochrome bc(1) complex via interactions with the Rieske iron-sulfur protein and cytochrome b in the ubiquinol oxidation pocket. As the targeted pathogens have developed resistance to this drug there is an urgent need for new alternatives. To better understand the determinants of inhibitor binding in the ubiquinol oxidation pocket of the bc(1) complex we synthesized a series of hydroxy-naphthoquinones bearing a methyl group on the benzene ring that is predicted to interact with the nuclear encoded Rieske iron-sulfur protein. We have also attempted to overcome the metabolic instability of a potent cytochrome bc(1) complex inhibitor, a 2-hydroxy-naphthoquinone with a branched side chain, by fluorinating the terminal methyl group. We have tested these new 2-hydroxy-naphthoquinones against yeast and bovine cytochrome bc(1) complexes to model the interaction with pathogen and human enzymes and determine parameters that affect efficacy of binding of these inhibitors. We identified a hydroxy-naphthoquinone with a trifluoromethyl function that has potential for development as an anti-fungal and anti-parasitic therapeutic.
Figures





Similar articles
-
Design of anti-parasitic and anti-fungal hydroxy-naphthoquinones that are less susceptible to drug resistance.Mol Biochem Parasitol. 2011 May;177(1):12-9. doi: 10.1016/j.molbiopara.2011.01.002. Epub 2011 Jan 18. Mol Biochem Parasitol. 2011. PMID: 21251932 Free PMC article.
-
Parameters determining the relative efficacy of hydroxy-naphthoquinone inhibitors of the cytochrome bc1 complex.Biochim Biophys Acta. 2007 Apr;1767(4):319-26. doi: 10.1016/j.bbabio.2007.02.014. Epub 2007 Feb 27. Biochim Biophys Acta. 2007. PMID: 17383607 Free PMC article.
-
Insights into cytochrome bc1 complex binding mode of antimalarial 2-hydroxy-1,4-naphthoquinones through molecular modelling.Mem Inst Oswaldo Cruz. 2017 Apr;112(4):299-308. doi: 10.1590/0074-02760160417. Mem Inst Oswaldo Cruz. 2017. PMID: 28327793 Free PMC article.
-
Modeling the molecular basis of atovaquone resistance in parasites and pathogenic fungi.Trends Parasitol. 2007 Oct;23(10):494-501. doi: 10.1016/j.pt.2007.08.004. Epub 2007 Sep 7. Trends Parasitol. 2007. PMID: 17826334 Review.
-
Are cytochrome b gene mutations the only cause of atovaquone resistance in Pneumocystis?Drug Resist Updat. 2001 Oct;4(5):322-9. doi: 10.1054/drup.2001.0221. Drug Resist Updat. 2001. PMID: 11991686 Review.
Cited by
-
Three-component reductive alkylation of 2-hydroxy-1, 4-naphthoquinones with lactols.Tetrahedron Lett. 2016 Feb 24;57(8):864-867. doi: 10.1016/j.tetlet.2016.01.036. Epub 2016 Jan 12. Tetrahedron Lett. 2016. PMID: 39105101 Free PMC article.
-
Biological evaluation of hydroxynaphthoquinones as anti-malarials.Malar J. 2013 Jul 10;12:234. doi: 10.1186/1475-2875-12-234. Malar J. 2013. PMID: 23841934 Free PMC article.
-
Phthalimide Derivatives with Bioactivity against Plasmodium falciparum: Synthesis, Evaluation, and Computational Studies Involving bc 1 Cytochrome Inhibition.ACS Omega. 2018 Aug 20;3(8):9424-9430. doi: 10.1021/acsomega.8b01062. eCollection 2018 Aug 31. ACS Omega. 2018. PMID: 31459076 Free PMC article.
-
Design of anti-parasitic and anti-fungal hydroxy-naphthoquinones that are less susceptible to drug resistance.Mol Biochem Parasitol. 2011 May;177(1):12-9. doi: 10.1016/j.molbiopara.2011.01.002. Epub 2011 Jan 18. Mol Biochem Parasitol. 2011. PMID: 21251932 Free PMC article.
-
Structural basis of resistance to anti-cytochrome bc₁ complex inhibitors: implication for drug improvement.Curr Pharm Des. 2014;20(5):704-24. doi: 10.2174/138161282005140214163327. Curr Pharm Des. 2014. PMID: 23688079 Free PMC article. Review.
References
-
- Vaidya AB, Mather MW. Atovaquone resistance in malaria parasites. Drug Resist. Update. 2000;3:283–287. - PubMed
-
- Kessl JJ, Lange BB, Merbitz-Zahradnick T, Zwicker K, Hill P, Meunier B, Meshnick S, Trumpower BL. Molecular basis for atovaquone binding to the cytochrome bc1 complex. J. Biol. Chem. 2003;278:31312–31318. - PubMed
-
- Srivastava IK, Morrisey JM, Darrouzet E, Daldal F, Vaidya AB. Resistance mutations reveal the atovaquone-binding domain of cytochrome b in malaria parasites. Mol. Microbiol. 1999;33:704–711. - PubMed
-
- Kessl JJ, Ha KH, Merritt AK, Lange BB, Hill P, Meunier B, Meshnick SR, Trumpower BL. Cytochrome b mutations that modify the ubiquinol-binding pocket of the cytochrome bc1 complex and confer anti-malarial drug resistance in Saccharomyces cerevisiae. J. Biol. Chem. 2005;280:17142–17148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

