Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity
- PMID: 19660437
- PMCID: PMC2784150
- DOI: 10.1016/j.bcp.2009.07.016
Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity
Abstract
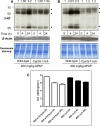
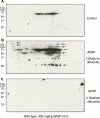
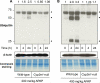
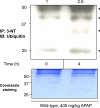
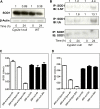
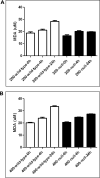
It is well established that following a toxic dose of acetaminophen (APAP), nitrotyrosine protein adducts (3-NT), a hallmark of peroxynitrite production, were colocalized with necrotic hepatic centrilobular regions where cytochrome P450 2E1 (CYP2E1) is highly expressed, suggesting that 3-NT formation may be essential in APAP-mediated toxicity. This study was aimed at investigating the relationship between CYP2E1 and nitration (3-NT formation) followed by ubiquitin-mediated degradation of proteins in wild-type and Cyp2e1-null mice exposed to APAP (200 and 400mg/kg) for 4 and 24h. Markedly increased centrilobular liver necrosis and 3-NT formation were only observed in APAP-exposed wild-type mice in a dose- and time-dependent manner, confirming an important role for CYP2E1 in APAP biotransformation and toxicity. However, the pattern of 3-NT protein adducts, not accompanied by concurrent activation of nitric oxide synthase (NOS), was similar to that of protein ubiquitination. Immunoblot analysis further revealed that immunoprecipitated nitrated proteins were ubiquitinated in APAP-exposed wild-type mice, confirming the fact that nitrated proteins are more susceptible than the native proteins for ubiquitin-dependent degradation, resulting in shorter half-lives. For instance, cytosolic superoxide dismutase (SOD1) levels were clearly decreased and immunoprecipitated SOD1 was nitrated and ubiquitinated, likely leading to its accelerated degradation in APAP-exposed wild-type mice. These data suggest that CYP2E1 appears to play a key role in 3-NT formation, protein degradation, and liver damage, which is independent of NOS, and that decreased levels of many proteins in the wild-type mice (compared with Cyp2e1-null mice) likely contribute to APAP-related toxicity.
Figures

References
-
- Thomas SH. Paracetamol (acetaminophen) poisoning. Pharmacol Ther. 1993;60:91–120. - PubMed
-
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. - PubMed
-
- James LP, Mayeux PR, Hinson JA. Acetaminophen-induced hepatotoxicity. Drug Metab Dispos. 2003;31:1499–506. - PubMed
-
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

