High frequency stimulation can block axonal conduction
- PMID: 19660453
- PMCID: PMC2761511
- DOI: 10.1016/j.expneurol.2009.07.023
High frequency stimulation can block axonal conduction
Abstract
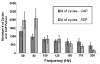
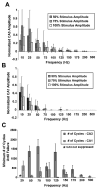
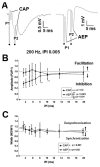
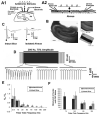
High frequency stimulation (HFS) is used to control abnormal neuronal activity associated with movement, seizure, and psychiatric disorders. Yet, the mechanisms of its therapeutic action are not known. Although experimental results have shown that HFS suppresses somatic activity, other data has suggested that HFS could generate excitation of axons. Moreover it is unclear what effect the stimulation has on tissue surrounding the stimulation electrode. Electrophysiological and computational modeling literature suggests that HFS can drive axons at the stimulus frequency. Therefore, we tested the hypothesis that unlike cell bodies, axons are driven by pulse train HFS. This hypothesis was tested in fibers of the hippocampus both in-vivo and in-vitro. Our results indicate that although electrical stimulation could activate and drive axons at low frequencies (0.5-25 Hz), as the stimulus frequency increased, electrical stimulation failed to continuously excite axonal activity. Fiber tracts were unable to follow extracellular pulse trains above 50 Hz in-vitro and above 125 Hz in-vivo. The number of cycles required for failure was frequency dependent but independent of stimulus amplitude. A novel in-vitro preparation was developed, in which, the alveus was isolated from the remainder of the hippocampus slice. The isolated fiber tract was unable to follow pulse trains above 75 Hz. Reversible conduction block occurred at much higher stimulus amplitudes, with pulse train HFS (>150 Hz) preventing propagation through the site of stimulation. This study shows that pulse train HFS affects axonal activity by: (1) disrupting HFS evoked excitation leading to partial conduction block of activity through the site of HFS; and (2) generating complete conduction block of secondary evoked activity, as HFS amplitude is increased. These results are relevant for the interpretation of the effects of HFS for the control of abnormal neural activity such as epilepsy and Parkinson's disease.
Figures






References
-
- Abelson J, Curtis G, Sagher O, Albucher R, Harrigan M, Taylor S, Martis B, Giordani B. Deep brain stimulation for refractory obsessive-compulsive disorder. Biological Psychiatry. 2005;57:510–516. - PubMed
-
- Bellinger S, Miyazawa A, Steinmetz P. Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: a modeling study. J Neural Engineering. 2008;5:263–274. - PubMed
-
- Benabid A, Benazzouz A, Hoffmann D, Limousin P, Krack P, Pollak P. Long-term electrical inhibition of deep brain targets in movement disorders. Movement Disorders. 1998;13:119–125. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

