Microsphere-based scaffolds for cartilage tissue engineering: using subcritical CO(2) as a sintering agent
- PMID: 19660579
- PMCID: PMC2787728
- DOI: 10.1016/j.actbio.2009.07.042
Microsphere-based scaffolds for cartilage tissue engineering: using subcritical CO(2) as a sintering agent
Abstract
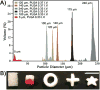
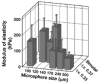
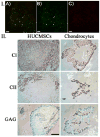
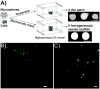
Shape-specific, macroporous tissue engineering scaffolds were fabricated and homogeneously seeded with cells in a single step. This method brings together CO(2) polymer processing and microparticle-based scaffolds in a manner that allows each to solve the key limitation of the other. Specifically, microparticle-based scaffolds have suffered from the limitation that conventional microsphere sintering methods (e.g., heat, solvents) are not cytocompatible, yet we have shown that cell viability was sustained with subcritical (i.e., gaseous) CO(2) sintering of microspheres in the presence of cells at near-ambient temperatures. On the other hand, the fused microspheres provided the pore interconnectivity that has eluded supercritical CO(2) foaming approaches. Here, fused poly(lactide-co-glycolide) microsphere scaffolds were seeded with human umbilical cord mesenchymal stromal cells to demonstrate the feasibility of utilizing these matrices for cartilage regeneration. We also demonstrated that the approach may be modified to produce thin cell-loaded patches as a promising alternative for skin tissue engineering applications.
Figures

Similar articles
-
Tailoring of processing parameters for sintering microsphere-based scaffolds with dense-phase carbon dioxide.J Biomed Mater Res B Appl Biomater. 2013 Feb;101(2):330-7. doi: 10.1002/jbm.b.32843. Epub 2012 Oct 31. J Biomed Mater Res B Appl Biomater. 2013. PMID: 23115065 Free PMC article.
-
Polymer-coated microparticle scaffolds engineered for potential use in musculoskeletal tissue regeneration.Biomed Mater. 2021 May 24;16(4):10.1088/1748-605X/abfdfd. doi: 10.1088/1748-605X/abfdfd. Biomed Mater. 2021. PMID: 33946056 Free PMC article.
-
Subcritical CO2 sintering of microspheres of different polymeric materials to fabricate scaffolds for tissue engineering.Mater Sci Eng C Mater Biol Appl. 2013 Dec 1;33(8):4892-9. doi: 10.1016/j.msec.2013.08.010. Epub 2013 Aug 15. Mater Sci Eng C Mater Biol Appl. 2013. PMID: 24094202 Free PMC article.
-
Mesenchymal stem cells for cartilage engineering.Biomed Mater Eng. 2012;22(1-3):69-80. doi: 10.3233/BME-2012-0691. Biomed Mater Eng. 2012. PMID: 22766704 Review.
-
Progress in microsphere-based scaffolds in bone/cartilage tissue engineering.Biomed Mater. 2023 Oct 6;18(6). doi: 10.1088/1748-605X/acfd78. Biomed Mater. 2023. PMID: 37751762 Review.
Cited by
-
Effect of Icariin on Engineered 3D-Printed Porous Scaffolds for Cartilage Repair.Materials (Basel). 2018 Aug 9;11(8):1390. doi: 10.3390/ma11081390. Materials (Basel). 2018. PMID: 30096899 Free PMC article.
-
Solvent-Free Approaches for the Processing of Scaffolds in Regenerative Medicine.Polymers (Basel). 2020 Mar 2;12(3):533. doi: 10.3390/polym12030533. Polymers (Basel). 2020. PMID: 32131405 Free PMC article. Review.
-
Microfluidic Chip Device for In Situ Mixing and Fabrication of Hydrogel Microspheres via Michael-Type Addition.Langmuir. 2021 Oct 12;37(40):11793-11803. doi: 10.1021/acs.langmuir.1c01739. Epub 2021 Oct 1. Langmuir. 2021. PMID: 34597052 Free PMC article.
-
The potential of encapsulating "raw materials" in 3D osteochondral gradient scaffolds.Biotechnol Bioeng. 2014 Apr;111(4):829-41. doi: 10.1002/bit.25145. Epub 2013 Nov 30. Biotechnol Bioeng. 2014. PMID: 24293388 Free PMC article.
-
Supercritical Fluid Technology: An Emphasis on Drug Delivery and Related Biomedical Applications.Adv Healthc Mater. 2017 Aug;6(16):10.1002/adhm.201700433. doi: 10.1002/adhm.201700433. Epub 2017 Jul 28. Adv Healthc Mater. 2017. PMID: 28752598 Free PMC article. Review.
References
-
- Chun KW, Yoo HS, Yoon JJ, Park TG. Biodegradable PLGA microcarriers for injectable delivery of chondrocytes: effect of surface modification on cell attachment and function. Biotechnology progress. 2004;20:1797–1801. - PubMed
-
- Holland TA, Tabata Y, Mikos AG. Dual growth factor delivery from degradable oligo(poly(ethylene glycol) fumarate) hydrogel scaffolds for cartilage tissue engineering. J Control Release. 2005;101:111–125. - PubMed
-
- Borden M, Attawia M, Khan Y, El-Amin SF, Laurencin CT. Tissue-engineered bone formation in vivo using a novel sintered polymeric microsphere matrix. J Bone Joint Surg Br. 2004;86:1200–1208. - PubMed
-
- Ruhe PQ, Hedberg-Dirk EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. Porous poly(DL-lactic-co-glycolic acid)/calcium phosphate cement composite for reconstruction of bone defects. Tissue Eng. 2006;12:789–800. - PubMed
-
- Goraltchouk A, Scanga V, Morshead CM, Shoichet MS. Incorporation of protein-eluting microspheres into biodegradable nerve guidance channels for controlled release. J Control Release. 2006;110:400–407. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

