Trophic factors therapy in Parkinson's disease
- PMID: 19660658
- PMCID: PMC3430519
- DOI: 10.1016/S0079-6123(09)17514-3
Trophic factors therapy in Parkinson's disease
Abstract
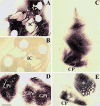
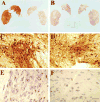
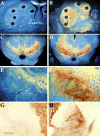
Parkinson's disease (PD) is a progressive, neurodegenerative disorder for which there is currently no effective neuroprotective therapy. Patients are typically treated with a combination of drug therapies and/or receive deep brain stimulation to combat behavioral symptoms. The ideal candidate therapy would be the one which prevents neurodegeneration in the brain, thereby halting the progression of debilitating disease symptoms. Neurotrophic factors have been in the forefront of PD research, and clinical trials have been initiated using members of the GDNF family of ligands (GFLs). GFLs have been shown to be trophic to ventral mesencephalic cells, thereby making them good candidates for PD research. This paper examines the use of GDNF and neurturin, two members of the GFL, in both animal models of PD and clinical trials.
Figures
References
-
- Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, et al. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. The Journal of Comparative Neurology. 2003;461:250–261. - PubMed
-
- Akerud P, Alberch J, Eketjall S, Wagner J, Arenas E. Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. Journal of Neurochemistry. 1999;73:70–78. - PubMed
-
- Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, et al. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8818–8823. - PMC - PubMed
-
- Burazin TC, Gundlach AL. Localization of GDNF/neurturin receptor (c-ret, GFRalpha-1 and alpha-2) mRNAs in postnatal rat brain: differential regional and temporal expression in hippocampus, cortex and cerebellum. Brain Research Molecular Brain Research. 1999;73:151–171. - PubMed
-
- Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

