Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1
- PMID: 19661057
- PMCID: PMC2781432
- DOI: 10.1074/jbc.M109.043455
Electrochemical measurement of electron transfer kinetics by Shewanella oneidensis MR-1
Abstract
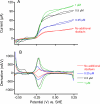
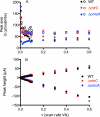
Shewanella oneidensis strain MR-1 can respire using carbon electrodes and metal oxyhydroxides as electron acceptors, requiring mechanisms for transferring electrons from the cell interior to surfaces located beyond the cell. Although purified outer membrane cytochromes will reduce both electrodes and metals, S. oneidensis also secretes flavins, which accelerate electron transfer to metals and electrodes. We developed techniques for detecting direct electron transfer by intact cells, using turnover and single turnover voltammetry. Metabolically active cells attached to graphite electrodes produced thin (submonolayer) films that demonstrated both catalytic and reversible electron transfer in the presence and absence of flavins. In the absence of soluble flavins, electron transfer occurred in a broad potential window centered at approximately 0 V (versus standard hydrogen electrode), and was altered in single (DeltaomcA, DeltamtrC) and double deletion (DeltaomcA/DeltamtrC) mutants of outer membrane cytochromes. The addition of soluble flavins at physiological concentrations significantly accelerated electron transfer and allowed catalytic electron transfer to occur at lower applied potentials (-0.2 V). Scan rate analysis indicated that rate constants for direct electron transfer were slower than those reported for pure cytochromes (approximately 1 s(-1)). These observations indicated that anodic current in the higher (>0 V) window is due to activation of a direct transfer mechanism, whereas electron transfer at lower potentials is enabled by flavins. The electrochemical dissection of these activities in living cells into two systems with characteristic midpoint potentials and kinetic behaviors explains prior observations and demonstrates the complementary nature of S. oneidensis electron transfer strategies.
Figures





References
-
- Richardson D. J. (2000) Microbiology 146, 551–571 - PubMed
-
- Nealson K. H., Saffarini D. (1994) Annu. Rev. Microbiol. 48, 311–343 - PubMed
-
- Lovley D. R., Holmes D. E., Nevin K. P. (2004) Adv. Microb. Physiol. 49, 219–286 - PubMed
-
- Nealson K. H., Scott J. (2005) in The Prokaryotes, pp. 1133–1151, Springer-Verlag New York Inc., New York
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

