Spectral domain optical coherence tomography in mouse models of retinal degeneration
- PMID: 19661229
- PMCID: PMC2800101
- DOI: 10.1167/iovs.09-3724
Spectral domain optical coherence tomography in mouse models of retinal degeneration
Abstract
Purpose: Spectral domain optical coherence tomography (SD-OCT) allows cross-sectional visualization of retinal structures in vivo. Here, the authors report the efficacy of a commercially available SD-OCT device to study mouse models of retinal degeneration.
Methods: C57BL/6 and BALB/c wild-type mice and three different mouse models of hereditary retinal degeneration (Rho(-/-), rd1, RPE65(-/-)) were investigated using confocal scanning laser ophthalmoscopy (cSLO) for en face visualization and SD-OCT for cross-sectional imaging of retinal structures. Histology was performed to correlate structural findings in SD-OCT with light microscopic data.
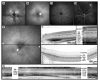
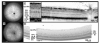
Results: In C57BL/6 and BALB/c mice, cSLO and SD-OCT imaging provided structural details of frequently used control animals (central retinal thickness, CRT(C57BL/6) = 237 +/- 2 microm and CRT(BALB/c) = 211 +/- 10 microm). RPE65(-/-) mice at 11 months of age showed a significant reduction of retinal thickness (CRT(RPE65) = 193 +/- 2 microm) with thinning of the outer nuclear layer. Rho(-/-) mice at P28 demonstrated degenerative changes mainly in the outer retinal layers (CRT(Rho) = 193 +/- 2 microm). Examining rd1 animals before and after the onset of retinal degeneration allowed monitoring of disease progression (CRT(rd1 P11) = 246 +/- 4 microm, CRT(rd1 P28) = 143 +/- 4 microm). Correlation of CRT assessed by histology and SD-OCT was high (r(2) = 0.897).
Conclusions: The authors demonstrated cross-sectional visualization of retinal structures in wild-type mice and mouse models for retinal degeneration in vivo using a commercially available SD-OCT device. This method will help to reduce numbers of animals needed per study by allowing longitudinal study designs and will facilitate characterization of disease dynamics and evaluation of putative therapeutic effects after experimental interventions.
Figures




Similar articles
-
Monitoring mouse retinal degeneration with high-resolution spectral-domain optical coherence tomography.J Vis. 2008 Jan 24;8(1):17.1-11. doi: 10.1167/8.1.17. J Vis. 2008. PMID: 18318620
-
Spectral-domain optical coherence tomography of the rodent eye: highlighting layers of the outer retina using signal averaging and comparison with histology.PLoS One. 2014 May 2;9(5):e96494. doi: 10.1371/journal.pone.0096494. eCollection 2014. PLoS One. 2014. PMID: 24788712 Free PMC article.
-
Long-term characterization of retinal degeneration in rd1 and rd10 mice using spectral domain optical coherence tomography.Invest Ophthalmol Vis Sci. 2012 Jul 10;53(8):4644-56. doi: 10.1167/iovs.12-9611. Invest Ophthalmol Vis Sci. 2012. PMID: 22562504 Free PMC article.
-
Optical Coherence Tomography of Animal Models of Retinitis Pigmentosa: From Animal Studies to Clinical Applications.Biomed Res Int. 2019 Oct 30;2019:8276140. doi: 10.1155/2019/8276140. eCollection 2019. Biomed Res Int. 2019. PMID: 31781647 Free PMC article. Review.
-
Spectral-domain optical coherence tomography: a comparison of modern high-resolution retinal imaging systems.Am J Ophthalmol. 2010 Jan;149(1):18-31. doi: 10.1016/j.ajo.2009.08.037. Am J Ophthalmol. 2010. PMID: 20103039 Review.
Cited by
-
Gene therapy in animal models of autosomal dominant retinitis pigmentosa.Mol Vis. 2012;18:2479-96. Epub 2012 Oct 6. Mol Vis. 2012. PMID: 23077406 Free PMC article. Review.
-
Correlation of in vivo and in vitro methods in measuring choroidal vascularization volumes using a subretinal injection induced choroidal neovascularization model.Chin Med J (Engl). 2015 Jun 5;128(11):1516-22. doi: 10.4103/0366-6999.157681. Chin Med J (Engl). 2015. PMID: 26021510 Free PMC article.
-
Fabricating customized hydrogel contact lens.Sci Rep. 2016 Oct 17;6:34905. doi: 10.1038/srep34905. Sci Rep. 2016. PMID: 27748361 Free PMC article.
-
Quantitative fundus autofluorescence in mice: correlation with HPLC quantitation of RPE lipofuscin and measurement of retina outer nuclear layer thickness.Invest Ophthalmol Vis Sci. 2013 Apr 17;54(4):2812-20. doi: 10.1167/iovs.12-11490. Invest Ophthalmol Vis Sci. 2013. PMID: 23548623 Free PMC article.
-
Conditional deletion of Des1 in the mouse retina does not impair the visual cycle in cones.FASEB J. 2019 Apr;33(4):5782-5792. doi: 10.1096/fj.201802493R. Epub 2019 Jan 15. FASEB J. 2019. PMID: 30645148 Free PMC article.
References
-
- Drexler W, Fujimoto JG. State-of-the-art retinal optical coherence tomography. Prog Retin Eye Res. 2008;27:45–88. - PubMed
-
- Horio N, Kachi S, Hori K, et al. Progressive change of optical coherence tomography scans in retinal degeneration slow mice. Arch Ophthalmol. 2001;119:1329–1332. - PubMed
-
- Li Q, Timmers AM, Hunter K, et al. Noninvasive imaging by optical coherence tomography to monitor retinal degeneration in the mouse. Invest Ophthalmol Vis Sci. 2001;42:2981–2989. - PubMed
-
- Anger EM, Unterhuber A, Hermann B, et al. Ultrahigh resolution optical coherence tomography of the monkey fovea. Identification of retinal sublayers by correlation with semithin histology sections. Exp Eye Res. 2004;78:1117–1125. - PubMed
-
- Kim KH, Puoris’haag M, Maguluri GN, et al. Monitoring mouse retinal degeneration with high-resolution spectral-domain optical coherence tomography. J Vis. 2008;8(17):11–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

