Role of cytosolic phospholipase A(2) in retinal neovascularization
- PMID: 19661235
- PMCID: PMC2868444
- DOI: 10.1167/iovs.09-3691
Role of cytosolic phospholipase A(2) in retinal neovascularization
Abstract
Purpose: To identify and characterize the role of cytosolic phospholipase A(2) (cPLA(2)) in retinal angiogenesis using relevant cell-based assays and a rodent model of retinopathy of prematurity.
Methods: The phosphorylation states of cPLA(2) and p38 MAP kinase and the expression of COX-2 were assessed by Western blot analysis in rat Müller cells. The activities of PLA(2) enzymes in rat retinal lysates were assessed using a commercially available assay. Prostaglandin E(2) (PGE(2)) and VEGF levels in Müller cell-conditioned medium and in retinal tissue samples were measured by ELISA. Rat retinal microvascular endothelial cell proliferation was measured using a BrdU assay. Efficacy of the cPLA(2) inhibitor CAY10502 was tested using the rat model of oxygen-induced retinopathy (OIR) in which neovascularization (NV) was assessed by computer-assisted image analysis.
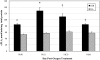
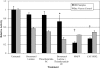
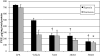
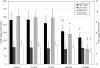
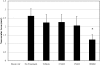
Results: In Müller cells, hypoxia increased the phosphorylation of cPLA(2) and p38 MAP kinase by 4-fold and 3-fold respectively. The cPLA(2) inhibitor CAY10502 decreased hypoxia-induced PGE(2) and VEGF levels in Müller cell-conditioned medium by 68.6% (P < 0.001) and 46.6% (P < 0.001), respectively. Retinal cPLA(2) activity peaked 1 day after oxygen exposure in OIR rats. CAY10502 (250 nM) decreased OIR-induced retinal PGE(2) and VEGF levels by 69% (P < 0.001) and 40.2% (P < 0.01), respectively. Intravitreal injection of 100 nM CAY10502 decreased retinal NV by 53.1% (P < 0.0001).
Conclusions: cPLA(2) liberates arachidonic acid, the substrate for prostaglandin (PG) production by the cyclooxygenase enzymes. PGs can exert a proangiogenic influence by inducing VEGF production and by stimulating angiogenic behaviors in vascular endothelial cells. Inhibition of cPLA(2) inhibits the production of proangiogenic PGs. Thus, cPLA(2) inhibition has a significant influence on pathologic retinal angiogenesis.
Figures

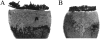
References
-
- Klagsbrun M. Regulators of angiogenesis: stimulators, inhibitors, and extracellular matrix. J Cell Biochem 1991; 47(3): 199–200 - PubMed
-
- Folkman J, Shing Y. Angiogenesis. J Biol Chem 1992; 267(16): 10931–10934 - PubMed
-
- Folkman J, D'Amore PA. Blood vessel formation: what is its molecular basis? Cell 1996; 87(7): 1153–1155 - PubMed
-
- Risau W. Mechanisms of angiogenesis. Nature 1997; 386(6626): 671–674 - PubMed
-
- Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech 2003; 60(1): 107–114 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

