Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness
- PMID: 19661246
- PMCID: PMC2778149
- DOI: 10.1164/rccm.200904-0573OC
Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness
Abstract
Rationale: In humans, immune responses to inhaled aeroallergens develop in the lung and draining lymph nodes. Many animal models of asthma bypass this route and instead use intraperitoneal injections of allergen using aluminum hydroxide as an adjuvant.
Objectives: We investigated whether allergic sensitization through the airway elicits immune responses qualitatively different than those arising in the peritoneum.
Methods: Mice were sensitized to allergen through the airway using low-dose LPS as an adjuvant, or through the peritoneum using aluminum hydroxide as an adjuvant. After a single allergen challenge, ELISA and flow cytometry were used to measure cytokines and leukocyte subsets. Invasive measurements of airway resistance were used to measure allergen-induced airway hyperreactivity (AHR).
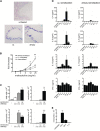
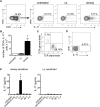
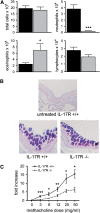
Measurements and main results: Sensitization through the peritoneum primed strong Th2 responses and eosinophilia, but not AHR, after a single allergen challenge. By contrast, allergic sensitization through the airway primed only modest Th2 responses, but strong Th17 responses. Th17 cells homed to the lung and released IL-17 into the airway on subsequent encounter with inhaled allergen. As a result, these mice developed IL-17-dependent airway neutrophilia and AHR. This AHR was neutrophil-dependent because it was abrogated in CXCR2-deficient mice and also in wild-type mice receiving a neutrophil-depleting antibody. Individually, neither IL-17 nor ongoing Th2 responses were sufficient to confer AHR, but together they acted synergistically to promote neutrophil recruitment, eosinophil recruitment and AHR.
Conclusions: Allergic sensitization through the airway primes modest Th2 responses but strong Th17 responses that promote airway neutrophilia and acute AHR. These findings support a causal role for neutrophils in severe asthma.
Figures
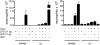


References
-
- Herrick CA, Bottomly K. To respond or not to respond: T cells in allergic asthma. Nat Rev Immunol 2003;3:405–412. - PubMed
-
- Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol 2003;111:450–463, quiz 464. - PubMed
-
- Holgate ST. Novel targets of therapy in asthma. Curr Opin Pulm Med 2009;15:63–71. - PubMed
-
- Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Page N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J Allergy Clin Immunol 2001;108:430–438. - PubMed
-
- Barnes PJ. New molecular targets for the treatment of neutrophilic diseases. J Allergy Clin Immunol 2007;119:1055–1062, quiz 1063–1064. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

