Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization
- PMID: 19661434
- PMCID: PMC2835775
- DOI: 10.1126/science.1171870
Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization
Abstract
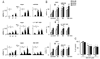
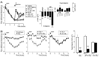
Diminished synaptic inhibition in the spinal dorsal horn is a major contributor to chronic pain. Pathways that reduce synaptic inhibition in inflammatory and neuropathic pain states have been identified, but central hyperalgesia and diminished dorsal horn synaptic inhibition also occur in the absence of inflammation or neuropathy, solely triggered by intense nociceptive (C-fiber) input to the spinal dorsal horn. We found that endocannabinoids, produced upon strong nociceptive stimulation, activated type 1 cannabinoid (CB1) receptors on inhibitory dorsal horn neurons to reduce the synaptic release of gamma-aminobutyric acid and glycine and thus rendered nociceptive neurons excitable by nonpainful stimuli. Our results suggest that spinal endocannabinoids and CB1 receptors on inhibitory dorsal horn interneurons act as mediators of heterosynaptic pain sensitization and play an unexpected role in dorsal horn pain-controlling circuits.
Figures


References
-
- Meyer R, Ringkamp M, Campbell J, Raja S. In: Textbook of Pain. McMahon SB, Koltzenburg M, editors. Churchill Livingstone: Elsevier; 2006. pp. 3–34.
-
- Sivilotti L, Woolf CJ. J Neurophysiol. 1994;72:169. - PubMed
-
- Yaksh TL. Pain. 1989;37:111. - PubMed
-
- Treede RD, Magerl W. Prog Brain Res. 2000;129:331. - PubMed
-
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Physiol Rev. 2009;89:309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

