Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA)
- PMID: 19661626
- PMCID: PMC2978421
- DOI: 10.3233/JAD-2009-1179
Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA)
Abstract
The Baltimore Longitudinal Study of Aging (BLSA) was established in 1958 and is one the oldest prospective studies of aging in the USA and the world. The BLSA is supported by the National Institute of Aging (NIA) and its mission is to learn what happens to people as they get old and how to sort out changes due to aging from those due to disease or other causes. In 1986, an autopsy program combined with comprehensive neurologic and cognitive evaluations was established in collaboration with the Johns Hopkins University Alzheimer's Disease Research Center (ADRC). Since then, 211 subjects have undergone autopsy. Here we review the key clinical neuropathological correlations from this autopsy series. The focus is on the morphological and biochemical changes that occur in normal aging, and the early neuropathological changes of neurodegenerative diseases, especially Alzheimer's disease (AD). We highlight the combined clinical, pathologic, morphometric, and biochemical evidence of asymptomatic AD, a state characterized by normal clinical evaluations in subjects with abundant AD pathology. We conclude that in some individuals, successful cognitive aging results from compensatory mechanisms that occur at the neuronal level (i.e., neuronal hypertrophy and synaptic plasticity) whereas a failure of compensation may culminate in disease.
Figures
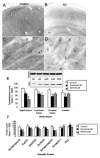

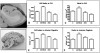
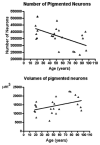
References
-
- Shock NW, Greulich RC, Costa PTJ, Andres R, Lakatta EG, Arenberg D, Tobin JD. Normal Human Aging: The Baltimore Longitudinal Study of Aging. U.S. Government Printing Office; Washington, D.C.: 1984.
-
- Kawas C, Gray S, Brookmeyer R, Fozard J, Zonderman A. Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology. 2000;54:2072–2077. - PubMed
-
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. - PubMed
-
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Thal LJ, Woodbury P. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology. 1997;48:1508–1510. - PubMed
-
- Silverman JM, Breitner JC, Mohs RC, Davis KL. Reliability of the family history method in genetic studies of Alzheimer’s disease and related dementias. Am J Psychiatry. 1986;143:1279–1282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

