Recognition of an intra-chain tandem 14-3-3 binding site within PKCepsilon
- PMID: 19662078
- PMCID: PMC2750047
- DOI: 10.1038/embor.2009.150
Recognition of an intra-chain tandem 14-3-3 binding site within PKCepsilon
Abstract
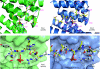
The phosphoserine/threonine binding protein 14-3-3 stimulates the catalytic activity of protein kinase C-epsilon (PKCepsilon) by engaging two tandem phosphoserine-containing motifs located between the PKCepsilon regulatory and catalytic domains (V3 region). Interaction between 14-3-3 and this region of PKCepsilon is essential for the completion of cytokinesis. Here, we report the crystal structure of 14-3-3zeta bound to a synthetic diphosphorylated PKCepsilon V3 region revealing how a consensus 14-3-3 site and a divergent 14-3-3 site cooperate to bind to 14-3-3 and so activate PKCepsilon. Thermodynamic data show a markedly enhanced binding affinity for two-site phosphopeptides over single-site 14-3-3 binding motifs and identifies Ser 368 as a gatekeeper phosphorylation site in this physiologically relevant 14-3-3 ligand. This dual-site intra-chain recognition has implications for other 14-3-3 targets, which seem to have only a single 14-3-3 motif, as other lower affinity and cryptic 14-3-3 gatekeeper sites might exist.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC (2002) PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr 58: 1948–1954 - PubMed
-
- Bridges D, Moorhead GB (2005) 14-3-3 proteins: a number of functions for a numbered protein. Sci STKE 2005: re10. - PubMed
-
- Delano WL (2002) The PyMOL Molecular Graphics System. Palo Alto, CA USA: Delano Scientific. http://www.pymol.org
-
- Durgan J, Cameron AJ, Saurin AT, Hanrahan S, Totty N, Messing RO, Parker PJ (2008) The identification and characterization of novel PKCepsilon phosphorylation sites provide evidence for functional cross-talk within the PKC superfamily. Biochem J 411: 319–331 - PubMed
-
- Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60: 2126–2132 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

