Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites
- PMID: 19662170
- PMCID: PMC2715215
- DOI: 10.1371/journal.ppat.1000542
Anopheles gambiae PGRPLC-mediated defense against bacteria modulates infections with malaria parasites
Abstract
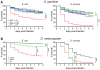
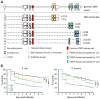
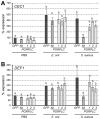
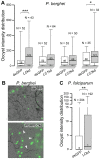
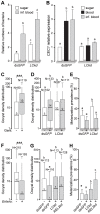
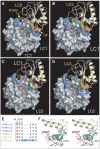
Recognition of peptidoglycan (PGN) is paramount for insect antibacterial defenses. In the fruit fly Drosophila melanogaster, the transmembrane PGN Recognition Protein LC (PGRP-LC) is a receptor of the Imd signaling pathway that is activated after infection with bacteria, mainly Gram-negative (Gram-). Here we demonstrate that bacterial infections of the malaria mosquito Anopheles gambiae are sensed by the orthologous PGRPLC protein which then activates a signaling pathway that involves the Rel/NF-kappaB transcription factor REL2. PGRPLC signaling leads to transcriptional induction of antimicrobial peptides at early stages of hemolymph infections with the Gram-positive (Gram+) bacterium Staphylococcus aureus, but a different signaling pathway might be used in infections with the Gram- bacterium Escherichia coli. The size of mosquito symbiotic bacteria populations and their dramatic proliferation after a bloodmeal, as well as intestinal bacterial infections, are also controlled by PGRPLC signaling. We show that this defense response modulates mosquito infection intensities with malaria parasites, both the rodent model parasite, Plasmodium berghei, and field isolates of the human parasite, Plasmodium falciparum. We propose that the tripartite interaction between mosquito microbial communities, PGRPLC-mediated antibacterial defense and infections with Plasmodium can be exploited in future interventions aiming to control malaria transmission. Molecular analysis and structural modeling provided mechanistic insights for the function of PGRPLC. Alternative splicing of PGRPLC transcripts produces three main isoforms, of which PGRPLC3 appears to have a key role in the resistance to bacteria and modulation of Plasmodium infections. Structural modeling indicates that PGRPLC3 is capable of binding monomeric PGN muropeptides but unable to initiate dimerization with other isoforms. A dual role of this isoform is hypothesized: it sequesters monomeric PGN dampening weak signals and locks other PGRPLC isoforms in binary immunostimulatory complexes further enhancing strong signals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Michel T, Reichhart JM, Hoffmann JA, Royet J. Drosophila Toll is activated by Gram-positive bacteria through a circulating peptidoglycan recognition protein. Nature. 2001;414:756–759. - PubMed
-
- Bischoff V, Vignal C, Boneca IG, Michel T, Hoffmann JA, et al. Function of the drosophila pattern-recognition receptor PGRP-SD in the detection of Gram-positive bacteria. Nat Immunol. 2004;5:1175–1180. - PubMed
-
- Gottar M, Gobert V, Michel T, Belvin M, Duyk G, et al. The Drosophila immune response against Gram-negative bacteria is mediated by a peptidoglycan recognition protein. Nature. 2002;416:640–644. - PubMed
-
- Choe KM, Werner T, Stoven S, Hultmark D, Anderson KV. Requirement for a peptidoglycan recognition protein (PGRP) in Relish activation and antibacterial immune responses in Drosophila. Science. 2002;296:359–362. - PubMed
-
- Kaneko T, Yano T, Aggarwal K, Lim JH, Ueda K, et al. PGRP-LC and PGRP-LE have essential yet distinct functions in the drosophila immune response to monomeric DAP-type peptidoglycan. Nat Immunol. 2006;7:715–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

