Biomarker discovery in animal health and disease: the application of post-genomic technologies
- PMID: 19662203
- PMCID: PMC2717813
Biomarker discovery in animal health and disease: the application of post-genomic technologies
Abstract
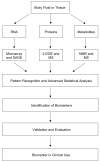
The causes of many important diseases in animals are complex and multifactorial, which present unique challenges. Biomarkers indicate the presence or extent of a biological process, which is directly linked to the clinical manifestations and outcome of a particular disease. Identifying biomarkers or biomarker profiles will be an important step towards disease characterization and management of disease in animals. The emergence of post-genomic technologies has led to the development of strategies aimed at identifying specific and sensitive biomarkers from the thousands of molecules present in a tissue or biological fluid. This review will summarize the current developments in biomarker discovery and will focus on the role of transcriptomics, proteomics and metabolomics in biomarker discovery for animal health and disease.
Keywords: Biomarker; Metabolomics; Proteomics; Transcriptomics; Veterinary.
Figures
Similar articles
-
Translational Metabolomics of Head Injury: Exploring Dysfunctional Cerebral Metabolism with Ex Vivo NMR Spectroscopy-Based Metabolite Quantification.In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. In: Kobeissy FH, editor. Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects. Boca Raton (FL): CRC Press/Taylor & Francis; 2015. Chapter 25. PMID: 26269925 Free Books & Documents. Review.
-
Multilevel omics for the discovery of biomarkers and therapeutic targets for stroke.Nat Rev Neurol. 2020 May;16(5):247-264. doi: 10.1038/s41582-020-0350-6. Epub 2020 Apr 22. Nat Rev Neurol. 2020. PMID: 32322099 Review.
-
Multiomics technologies: role in disease biomarker discoveries and therapeutics.Brief Funct Genomics. 2023 Apr 13;22(2):76-96. doi: 10.1093/bfgp/elac017. Brief Funct Genomics. 2023. PMID: 35809340 Review.
-
The application of mass spectrometry to proteomics and metabolomics in biomarker discovery and drug development.Curr Mol Pharmacol. 2012 Jun;5(2):301-16. doi: 10.2174/1874467211205020301. Curr Mol Pharmacol. 2012. PMID: 22122469 Review.
-
The Future of Biomarkers in Veterinary Medicine: Emerging Approaches and Associated Challenges.Animals (Basel). 2022 Aug 26;12(17):2194. doi: 10.3390/ani12172194. Animals (Basel). 2022. PMID: 36077913 Free PMC article. Review.
Cited by
-
Integrating (Nutri-)Metabolomics into the One Health Tendency-The Key for Personalized Medicine Advancement.Metabolites. 2023 Jun 27;13(7):800. doi: 10.3390/metabo13070800. Metabolites. 2023. PMID: 37512507 Free PMC article. Review.
-
BioKA: a curated and integrated biomarker knowledgebase for animals.Nucleic Acids Res. 2024 Jan 5;52(D1):D1121-D1130. doi: 10.1093/nar/gkad873. Nucleic Acids Res. 2024. PMID: 37843156 Free PMC article.
-
Analysis of mass spectrometry data from the secretome of an explant model of articular cartilage exposed to pro-inflammatory and anti-inflammatory stimuli using machine learning.BMC Musculoskelet Disord. 2013 Dec 13;14:349. doi: 10.1186/1471-2474-14-349. BMC Musculoskelet Disord. 2013. PMID: 24330474 Free PMC article.
-
Analysis of metabolomic patterns in thoroughbreds before and after exercise.Asian-Australas J Anim Sci. 2017 Nov;30(11):1633-1642. doi: 10.5713/ajas.17.0167. Epub 2017 Jun 27. Asian-Australas J Anim Sci. 2017. PMID: 28728374 Free PMC article.
-
Multi Platforms Strategies and Metabolomics Approaches for the Investigation of Comprehensive Metabolite Profile in Dogs with Babesia canis Infection.Int J Mol Sci. 2022 Jan 29;23(3):1575. doi: 10.3390/ijms23031575. Int J Mol Sci. 2022. PMID: 35163517 Free PMC article.
References
-
- Abdul-Careem MF, Hunter BD, Nagy E, et al. Development of a real-time PCR assay using SYBR Green chemistry for monitoring Marek’s disease virus genome load in feather tips. J Virol Methods. 2006;133:34–40. - PubMed
-
- Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003;422:198–207. - PubMed
-
- Anderson NL, Anderson NG. The roles of multiple proteomic platforms in a pipeline for new diagnostics. Mol Cell Proteomics. 2005;4:1441–4. - PubMed
-
- Baggerly KA, Morris JS, Coombes KR. Reproducibility of SELDI-ToF protein patterns in serum: comparing datasets from different experiments. Bioinformatics. 2004;20:777–85. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
