Protein Tyrosine Phosphatase Gamma (PTPgamma) is a Novel Leukocyte Marker Highly Expressed by CD34 Precursors
- PMID: 19662205
- PMCID: PMC2717823
Protein Tyrosine Phosphatase Gamma (PTPgamma) is a Novel Leukocyte Marker Highly Expressed by CD34 Precursors
Abstract
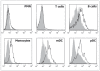
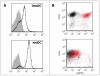
Protein Tyrosine Phosphatase gamma (PTPgamma) is a receptor-like transmembrane protein belonging to the family of classical protein tyrosine phosphatases. PTPgamma is known to regulate haematopoietic differentiation in a murine embryonic stem cells model. We have recently demonstrated that PTPgamma mRNA is expressed in monocytes, tissue-localized myeloid dendritic cells and in both myeloid and plasmacytoid dendritic cells in peripheral blood. We now developed a PTPgamma specific antibody that recognizes the protein by flow cytometry. PTPgamma expression was detected in monocytes and both myeloid and plasmacytoid dendritic cells, while PMN showed a low but consistent staining in contrast with previous mRNA data. B cells were found to express the phosphatase while T cells were negative. In keeping with RNA data, PTPgamma was detected in monocyte-derived dendritic cells and its expression rose upon LPS stimulation. Finally, we discovered that CD34(+) haematopoietic precursors express high PTPgamma level that drops during in vitro expansion induced by IL-3 and SCF growth factors. We therefore propose PTPgamma as a new functionally regulated leukocyte marker whose role in normal and pathological context deserve further investigation.
Keywords: Egg yolk immunoglobulin (IgY); fluorescence activated cell sorting (FACS); haematopoietic progenitors; human protein tyrosine phosphatase gamma (PTPγ).
Figures

Similar articles
-
Receptor-type protein tyrosine phosphatase gamma (PTPgamma), a new identifier for myeloid dendritic cells and specialized macrophages.Blood. 2006 Dec 15;108(13):4223-31. doi: 10.1182/blood-2006-05-024257. Epub 2006 Aug 8. Blood. 2006. PMID: 16896153 Clinical Trial.
-
Involvement of breast epithelial-stromal interactions in the regulation of protein tyrosine phosphatase-gamma (PTPgamma) mRNA expression by estrogenically active agents.Breast Cancer Res Treat. 2002 Jan;71(1):21-35. doi: 10.1023/a:1013343718942. Breast Cancer Res Treat. 2002. PMID: 11859871
-
Expression of transmembrane protein tyrosine phosphatase gamma (PTPgamma) in normal and neoplastic human tissues.Histopathology. 2007 Apr;50(5):615-28. doi: 10.1111/j.1365-2559.2007.02661.x. Histopathology. 2007. PMID: 17394498
-
Expression of FLT3 receptor and response to FLT3 ligand by leukemic cells.Leukemia. 1996 Apr;10(4):588-99. Leukemia. 1996. PMID: 8618433 Review.
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
Cited by
-
Predictive value of tyrosine phosphatase receptor gamma for the response to treatment tyrosine kinase inhibitors in chronic myeloid leukemia patients.Sci Rep. 2021 Apr 23;11(1):8833. doi: 10.1038/s41598-021-86875-y. Sci Rep. 2021. PMID: 33893334 Free PMC article.
-
Novel RPTPγ and RPTPζ splice variants from mixed neuron-astrocyte hippocampal cultures as well as from the hippocampi of newborn and adult mice.Front Physiol. 2024 Jun 17;15:1406448. doi: 10.3389/fphys.2024.1406448. eCollection 2024. Front Physiol. 2024. PMID: 38952869 Free PMC article.
-
A new monoclonal antibody detects downregulation of protein tyrosine phosphatase receptor type γ in chronic myeloid leukemia patients.J Hematol Oncol. 2017 Jun 21;10(1):129. doi: 10.1186/s13045-017-0494-z. J Hematol Oncol. 2017. PMID: 28637510 Free PMC article.
-
Identification of protein tyrosine phosphatase receptor gamma extracellular domain (sPTPRG) as a natural soluble protein in plasma.PLoS One. 2015 Mar 16;10(3):e0119110. doi: 10.1371/journal.pone.0119110. eCollection 2015. PLoS One. 2015. PMID: 25775014 Free PMC article.
-
Exploring cellular changes in ruptured human quadriceps tendons at single-cell resolution.J Physiol. 2025 Aug;603(16):4535-4554. doi: 10.1113/JP287812. Epub 2025 Apr 15. J Physiol. 2025. PMID: 40232153 Free PMC article.
References
-
- Andersson LC, Nilsson K, Gahmberg CG. K562—a human erythroleukemic cell line. Int J Cancer. 1979;23:143–7. - PubMed
-
- Barnea G, Silvennoinen O, Shaanan B, Honegger AM, Canoll PD, D’eustachio P, Morse B, Levy JB, Laforgia S, Huebner K, et al. Identification of a carbonic anhydrase-like domain in the extracellular region of RPTP gamma defines a new subfamily of receptor tyrosine phosphatases. Mol Cell Biol. 1993;13:1497–506. - PMC - PubMed
-
- Gallagher R, Collins S, Trujillo J, Mccredie K, Ahearn M, Tsai S, Metzgar R, Aulakh G, Ting R, Ruscetti F, Gallo R. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–33. - PubMed
-
- Kappert K, Peters KG, Bohmer FD, Ostman A. Tyrosine phosphatases in vessel wall signaling. Cardiovasc Res. 2005;65:587–98. - PubMed
LinkOut - more resources
Full Text Sources
