Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia
- PMID: 19662681
- PMCID: PMC2719928
- DOI: 10.1172/jci38879
Neonatal NK cells target the mouse duct epithelium via Nkg2d and drive tissue-specific injury in experimental biliary atresia
Abstract
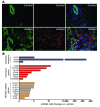
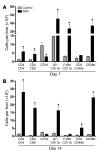
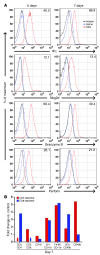
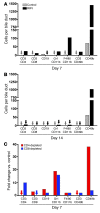
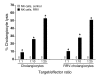
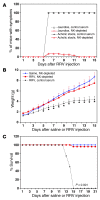
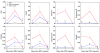
Biliary atresia is a neonatal obstructive cholangiopathy that progresses to end-stage liver disease. Although the etiology is unknown, a neonatal adaptive immune signature has been mechanistically linked to obstruction of the extrahepatic bile ducts. Here, we investigated the role of the innate immune response in the pathogenesis of biliary atresia. Analysis of livers of infants at diagnosis revealed that NK cells populate the vicinity of intrahepatic bile ducts and overexpress several genes involved in cytotoxicity. Using a model of rotavirus-induced biliary atresia in newborn mice, we found that activated NK cells also populated murine livers and were the most abundant cells in extrahepatic bile ducts at the time of obstruction. Rotavirus-primed hepatic NK cells lysed cholangiocytes in a contact- and Nkg2d-dependent fashion. Depletion of NK cells and blockade of Nkg2d each prevented injury of the duct epithelium after rotavirus infection, maintained continuity of duct lumen between the liver and duodenum, and enabled bile flow, despite the presence of virus in the tissue and the overexpression of proinflammatory cytokines. These findings identify NK cells as key initiators of cholangiocyte injury via Nkg2d and demonstrate that injury to the duct epithelium drives the phenotype of experimental biliary atresia.
Figures



Comment in
-
Cooperation of innate and adaptive immunity in the pathogenesis of biliary atresia: there's a killer on the run.Hepatology. 2009 Dec;50(6):2037-40. doi: 10.1002/hep.23399. Hepatology. 2009. PMID: 19937699 No abstract available.
References
-
- Bezerra J.A., et al. Genetic induction of proinflammatory immunity in children with biliary atresia. Lancet. 2002;360:1653–1659. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

