Trapping an intermediate of dinitrogen (N2) reduction on nitrogenase
- PMID: 19663502
- PMCID: PMC2814451
- DOI: 10.1021/bi901092z
Trapping an intermediate of dinitrogen (N2) reduction on nitrogenase
Abstract
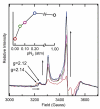
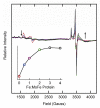
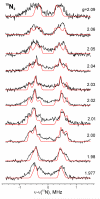
Nitrogenase reduces dinitrogen (N2) by six electrons and six protons at an active-site metallocluster called FeMo cofactor, to yield two ammonia molecules. Insights into the mechanism of substrate reduction by nitrogenase have come from recent successes in trapping and characterizing intermediates generated during the reduction of protons as well as nitrogenous and alkyne substrates by MoFe proteins with amino acid substitutions. Here, we describe an intermediate generated at a high concentration during reduction of the natural nitrogenase substrate, N2, by wild-type MoFe protein, providing evidence that it contains N2 bound to the active-site FeMo cofactor. When MoFe protein was frozen at 77 K during steady-state turnover with N2, the S = 3/2 EPR signal (g = [4.3, 3.64, 2.00]) arising from the resting state of FeMo cofactor was observed to convert to a rhombic, S = 1/2, signal (g = [2.08, 1.99, 1.97]). The intensity of the N2-dependent EPR signal increased with increasing N2 partial pressure, reaching a maximum intensity of approximately 20% of that of the original FeMo cofactor signal at > or = 0.2 atm N2. An almost complete loss of resting FeMo cofactor signal in this sample implies that the remainder of the enzyme has been reduced to an EPR-silent intermediate state. The N2-dependent EPR signal intensity also varied with the ratio of Fe protein to MoFe protein (electron flux through nitrogenase), with the maximum signal intensity observed with a ratio of 2:1 (1:1 Fe protein:FeMo cofactor) or higher. The pH optimum for the signal was 7.1. The N2-dependent EPR signal intensity exhibited a linear dependence on the square root of the EPR microwave power in contrast to the nonlinear response of signal intensity observed for hydrazine-, diazene-, and methyldiazene-trapped states. 15N ENDOR spectroscopic analysis of MoFe protein captured during turnover with 15N2 revealed a 15N nuclear spin coupled to the FeMo cofactor with a hyperfine tensor A = [0.9, 1.4, 0.45] MHz establishing that an N2-derived species was trapped on the FeMo cofactor. The observation of a single type of 15N-coupled nucleus from the field dependence, along with the absence of an associated exchangeable 1H ENDOR signal, is consistent with an N2 molecule bound end-on to the FeMo cofactor.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

