The role of T helper cell differentiation in promoting nerve allograft survival with costimulation blockade
- PMID: 19663546
- PMCID: PMC2956431
- DOI: 10.3171/2009.7.JNS09187
The role of T helper cell differentiation in promoting nerve allograft survival with costimulation blockade
Abstract
Object: Peripheral nerve allografts provide a temporary scaffold for host nerve regeneration and allow for the repair of significant segmental nerve injuries. Despite this potential, nerve allograft transplantation requires temporary systemic immunosuppression. Characterization of the immunological mechanisms involved in the induction of immune hyporesponsiveness to prevent nerve allograft rejection will help provide a basis for optimizing immunomodulation regimens or manipulating donor nerve allografts to minimize or eliminate the need for global immunosuppression.
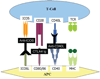
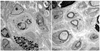
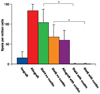
Methods: The authors used C57Bl/6 mice and STAT4 and STAT6 gene BALB/c knockout mice. A nonvascularized nerve allograft was used to reconstruct a 1-cm sciatic nerve gap in the murine model. A triple costimulatory blockade of the CD40, CD28/B7, and inducible costimulatory (ICOS) pathways was used. Quantitative assessment was performed at 3 weeks with nerve histomorphometry, walking track analysis, and the enzyme-linked immunospot assay.
Results: The STAT6 -/- mice received 3 doses of costimulation-blocking antibodies and had axonal regeneration equivalent to nerve isografts, while treated STAT4 -/- mice demonstrated moderate axonal regeneration but inferior to the T helper cell Type 2-deficient animals. Enzyme-linked immunospot assay analysis demonstrated a minimal immune response in both STAT4 -/- and STAT6 -/- mice treated with a costimulatory blockade.
Conclusions: The authors' findings suggest that Type 1 T helper cells may play a more significant role in costimulatory blockade-induced immune hyporesponsiveness in the nerve allograft model, and that Type 2 T helper differentation may represent a potential target for directed immunosuppression.
Figures




References
-
- Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. - PubMed
-
- Bluestone JA. New perspectives of CD28-B7-mediated T cell costimulation. Immunity. 1995;2:555–559. - PubMed
-
- Brenner MJ, Tung TH, Mackinnon SE, Myckatyn TM, Hunter DA, Mohanakumar T. Anti-CD40 ligand monoclonal antibody induces a permissive state, but not tolerance, for murine peripheral nerve allografts. Exp Neurol. 2004;186:59–69. - PubMed
-
- Brown DL, Bishop DK, Wood SY, Cederna PS. Short-term anti-CD40 ligand costimulatory blockade induces tolerance to peripheral nerve allografts, resulting in improved skeletal muscle function. Plast Reconstr Surg. 2006;117:2250–2258. - PubMed
-
- Buttemeyer R, Rao U, Jones NF. Peripheral nerve allograft transplantation with FK506: functional, histological, and immunological results before and after discontinuation of immunosuppression. Ann Plast Surg. 1995;35:396–401. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

