CYP2C9*8 is prevalent among African-Americans: implications for pharmacogenetic dosing
- PMID: 19663669
- PMCID: PMC2737687
- DOI: 10.2217/pgs.09.71
CYP2C9*8 is prevalent among African-Americans: implications for pharmacogenetic dosing
Abstract
Aims: Although the frequencies of pharmacogenetic variants differ among racial groups, most pharmacogenetic algorithms for genotype-guided warfarin dosing only include two CYP2C9 alleles (*2 and *3) and a single VKORC1 allele (g.-1639G>A or g.1173C>T) commonly found among Caucasians. Therefore, this study sought to identify other CYP2C9 and VKORC1 alleles important in warfarin dose variability and to determine their frequencies in different racial and ethnic groups.
Materials & methods: The CYP2C9 and VKORC1 genes were sequenced in selected sensitive (< 21 mg/week) and resistant (> 49 mg/week) individuals with discrepant therapeutic and algorithm-predicted warfarin doses based on prior CYP2C9 and VKORC1 genotyping. The CYP2C9 and VKORC1 allele frequencies were determined in healthy, racially self-identified blood donors.
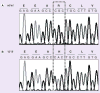
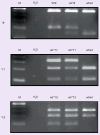
Results: Sequencing identified an African-American male with a lower than predicted therapeutic warfarin dose (14.4 mg/week), previously genotyped as CYP2C9*1/*1, who was homozygous for CYP2C9*8 (c.449G>A; p.R150H). Genotyping 600 African-American alleles identified CYP2C9*8 as their most frequent variant CYP2C9 allele (0.047), and the combined allele frequency of CYP2C9*2, *3, *5, *6, *8 and *11 was 0.133. Given most warfarin pharmacogenetic dosing algorithms only include CYP2C9*2 and *3, the inclusion of CYP2C9*8 alone could reclassify the predicted metabolic phenotypes of almost 10% of African-Americans, or when combined with CYP2C9*5, *6 and *11, more than 15%. In addition, the African-American VKORC1 g.-1639A allele frequency was 0.108 and three g.1331G>A (p.V66M) carriers were identified.
Conclusions: CYP2C9*8 is prevalent among African-Americans ( approximately 1 in 11 individuals). Thus, in this racial group, the incorporation of CYP2C9*8 into genotyping panels may improve dose prediction of CYP2C9-metabolized drugs, including warfarin.
Figures
References
Bibliography
-
- Sim SC, Ingelman-Sundberg M. The human cytochrome P450 Allele Nomenclature Committee Web site: submission criteria, procedures, and objectives. Methods Mol Biol. 2006;320:183–191. - PubMed
-
- Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA. 2002;287:1690–1698. - PubMed
-
- Aquilante CL, Langaee TY, Lopez LM, et al. Influence of coagulation factor, vitamin K epoxide reductase complex subunit 1, and cytochrome P450 2C9 gene polymorphisms on warfarin dose requirements. Clin Pharmacol Ther. 2006;79:291–302. - PubMed
-
- Carlquist JF, Horne BD, Muhlestein JB, et al. Genotypes of the cytochrome p450 isoform, CYP2C9, and the vitamin K epoxide reductase complex subunit 1 conjointly determine stable warfarin dose: a prospective study. J Thromb Thrombolysis. 2006;22:191–197. - PubMed
-
- Takahashi H, Wilkinson GR, Nutescu EA, et al. Different contributions of polymorphisms in VKORC1 and CYP2C9 to intra- and inter-population differences in maintenance dose of warfarin in Japanese, Caucasians and African–Americans. Pharmacogenet Genomics. 2006;16:101–110. - PubMed
Website
-
- Home Page of the Human Cytochrome P450 (CYP) Allele Nomenclature Committee . www.cypalleles.ki.se/cyp2c9.htm.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
