NMR and molecular modelling studies on the interaction of fluconazole with beta-cyclodextrin
- PMID: 19664263
- PMCID: PMC2739844
- DOI: 10.1186/1752-153X-3-9
NMR and molecular modelling studies on the interaction of fluconazole with beta-cyclodextrin
Abstract
Background: Fluconazole (FLZ) is a synthetic, bistriazole antifungal agent, effective in treating superficial and systemic infections caused by Candida species. Major challenges in formulating this drug for clinical applications include solubility enhancement and improving stability in biological systems. Cyclodextrins (CDs) are chiral, truncated cone shaped macrocyles, and can easily encapsulate fluconazole inside their hydrophobic cavity. NMR spectroscopy has been recognized as an important tool for the interaction study of cyclodextrin and pharmaceutical compounds in solution state.
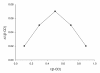
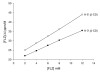
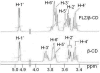
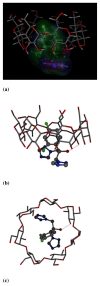
Results: Inclusion complex of fluconazole with beta-cyclodextrins (beta-CD) were investigated by applying NMR and molecular modelling methods. The 1:1 stoichiometry of FLZ:beta-CD complex was determined by continuous variation (Job's plot) method and the overall association constant was determined by using Scott's method. The association constant was determined to be 68.7 M-1 which is consistent with efficient FLZ:beta-CD complexation. The shielding of cavity protons of beta-CD and deshielding of aromatic protons of FLZ in various 1H-NMR experiments show complexation between beta-CD and FLZ. Based on spectral data obtained from 2D ROESY, a reasonable geometry for the complex could be proposed implicating the insertion of the m-difluorophenyl ring of FLZ into the wide end of the torus cavity of beta-CD. Molecular modelling studies were conducted to further interpret the NMR data. Indeed the best docked complex in terms of binding free energy supports the model proposed from NMR experiments and the m-difluorophenyl ring of FLZ is observed to enter into the torus cavity of beta-CD from the wider end.
Conclusion: Various NMR spectroscopic studies of FLZ in the presence of beta-CD in D2O at room temperature confirmed the formation of a 1:1 (FLZ:beta-CD) inclusion complex in which m-difluorophenyl ring acts as guest. The induced shift changes as well as splitting of most of the signals of FLZ in the presence of beta-CD suggest some chiral differentiation of guest by beta-CD.
Figures




References
-
- Meunier F, Aoun M, Gerard M. Therapy for oropharyngeal candidiasis in the immunocompromised host: a randomized double-blind study of fluconazole vs. ketoconazole. Rev Infect Dis. 1990;12:S364–S368. - PubMed
-
- MacMillan ML, Goodman JL, DeFor TE, Weisdorf DJ. Fluconazole to prevent yeast infections in bone marrow transplantation patients: a randomized trial of high versus reduced dose, and determination of the value of maintenance therapy. Am J Med. 2002;112:369–379. doi: 10.1016/S0002-9343(01)01127-5. - DOI - PubMed
-
- Dodziuk H. Cyclodextrins and Their Complexes. Chemistry, Analytical Methods, Applications. Wiley-VCH: London; 2006.
LinkOut - more resources
Full Text Sources

