The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties
- PMID: 19664287
- PMCID: PMC2745803
- DOI: 10.1186/ar2783
The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties
Erratum in
- Arthritis Res Ther. 2009;11(5):415
Abstract
Introduction: The Fibromyalgia Impact Questionnaire (FIQ) is a commonly used instrument in the evaluation of fibromyalgia (FM) patients. Over the last 18 years, since the publication of the original FIQ, several deficiencies have become apparent and the cumbersome scoring algorithm has been a barrier to widespread clinical use. The aim of this paper is to describe and validate a revised version of the FIQ: the FIQR.
Methods: The FIQR was developed in response to known deficiencies of the FIQ with the help of a patient focus group. The FIQR has the same 3 domains as the FIQ (that is, function, overall impact and symptoms). It differs from the FIQ in having modified function questions and the inclusion of questions on memory, tenderness, balance and environmental sensitivity. All questions are graded on a 0-10 numeric scale. The FIQR was administered online and the results were compared to the same patient's online responses to the 36-Item Short Form Health Survey (SF-36) and the original FIQ.
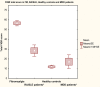
Results: The FIQR was completed online by 202 FM patients, 51 rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE) patients (31 RA and 20 SLE), 11 patients with major depressive disorder (MDD) and 213 healthy controls (HC). The mean total FIQR score was 56.6 +/- 19.9 compared to a total FIQ score of 60.6 +/- 17.8 (P < 0.03). The total scores of the FIQR and FIQ were closely correlated (r = 0.88, P < 0.001). Each of the 3 domains of the FIQR correlated well with the 3 related FIQ domains (r = 0.69 to 0.88, P < 0.01). The FIQR showed good correlation with comparable domains in the SF-36, with a multiple regression analysis showing that the three FIQR domain scores predicted the 8 SF-36 subscale scores. The FIQR had good discriminant ability between FM and the 3 other groups; total FIQR scores were HC (12.1 +/- 11.6), RA/SLE (28.6 +/- 21.2) and MDD (17.3 +/- 11.8). The patient completion time was 1.3 minutes; scoring took about 1 minute.
Conclusions: The FIQR is an updated version of the FIQ that has good psychometric properties, can be completed in less than 2 minutes and is easy to score. It has scoring characteristics comparable to the original FIQ, making it possible to compare past FIQ results with future FIQR results.
Figures


References
-
- Burckhardt CS, Clark BD, Bennett RM. The fibromyalgia impact questionnaire: development and validation. J Rheumatol. 1991;18:728–733. - PubMed
-
- Bennett R. The Fibromyalgia Impact Questionnaire (FIQ): a review of its development, current version, operating characteristics and uses. Clin Exp Rheumatol. 2005;23:S154–S162. - PubMed
-
- Dunkl PR, Taylor AG, McConnell GG, Alfano AP, Conaway MR. Responsiveness of fibromyalgia clinical trial outcome measures. J Rheumatol. 2000;27:2683–2691. - PubMed
-
- Prodinger B, Cieza A, Williams DA, Mease P, Boonen A, Kerschan-Schindl K, Fialka-Moser V, Smolen J, Stucki G, Machold K, Stamm T. Measuring health in patients with fibromyalgia: content comparison of questionnaires based on the International Classification of Functioning, Disability and Health. Arthritis Rheum. 2008;59:650–658. doi: 10.1002/art.23559. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

